Chemistry Titration Worksheet
Is a reaction between and acid and a base that produces a.
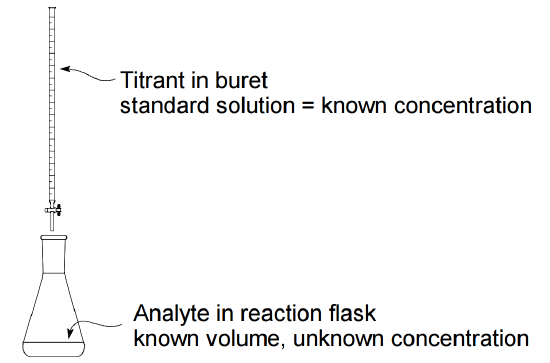
Chemistry titration worksheet. Neutralization reactions go to completion. Ap stoichiometry 1. Ap acids and bases 12. Be sure to write the neutralization reaction 6.
What is the molarity of the acid. Empirical formula and percent composition. 100 ml of 100 m hcl neutralized 200 ml of a naoh solution. A worksheet on titration calculations and percentage uncertainties.
Therefore learning how to calculate the ph throughout the course of a titration is a useful way of bringing all of the material we have studied together in a meaningful way. 2 if it takes 25 ml of 005 m hcl to neutralize 345 ml of naoh solution what is the concentration of the naoh solution. Ap acids and bases 11. Worksheet solutions by unit.
This website and its content is subject to our terms and conditions. What was the molarity of the hcl. What was the molarity of the naoh. Titration worksheet name.
Chapter 16 worksheet 2 ws162 understanding the shapes of acid base titration curves. Every acid base reaction produces another acid and another base. In a titration of h 2so 4 with naoh 600 ml of 0020 m naoh was needed to neutralize 150 ml of h 2so 4. Titrations worksheet w 336 everett community college tutoring center student support services program 1 it takes 83 ml of a 045 m naoh solution to neutralize 235 ml of an hcl solution.
More acids and bases. Titrations practice worksheet find the requested quantities in the following problems. In a titration of hcl with naoh 1000 ml of the base was required to neutralize 200 ml of 50 m hcl. Ap acids and bases 13.
Titrations and titration calculations are tricky topics that many gcse chemistry students find difficult. Show all work for any credit. This dedicated titrations page will cover how to carry out a titration and how to perform titration calculations in line with the gcse chemistry syllabus. 1 if it takes 54 ml of 01 m naoh to neutralize 125 ml of an hcl solution what is the concentration of the hcl.
Consider the titration of an acetic acid solution with a sodium hydroxide solution at the following three stages of the titration. 120 ml of 500 m naoh neutralized 60 ml of hcl solution.


















