Clinical Development Plan Template

49 target product profile typically.
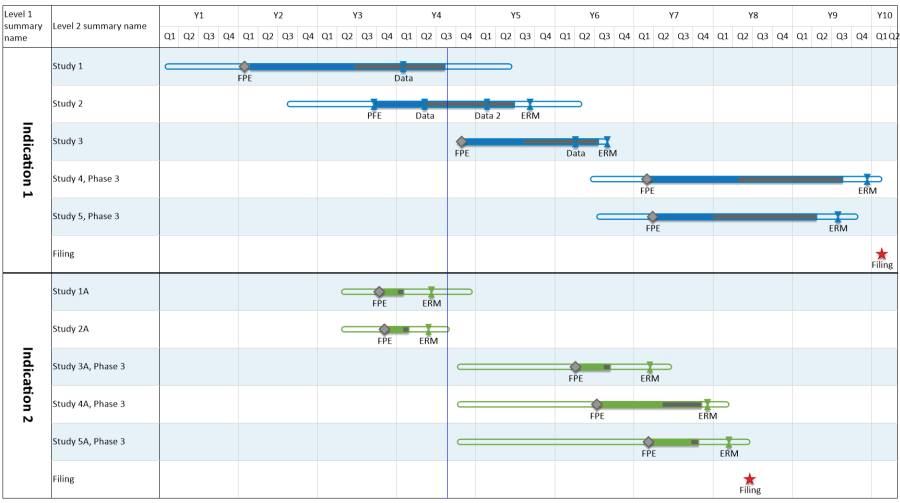
Clinical development plan template. The cdp should not to be confused with the fdas ind section entitled general investigational plan which is a short summary of the trials planned for the upcoming year the cdp is an internal corporate management document. Forms the basis of the clinical development plan cdpand probably all other dps and draft label. Description of product with evidence or speculation of effect in. The clinical development plan is a complex document that entails the entire clinical research strategy of a drug describing the clinical studies that will be carried out for a pharmaceutical entity created by a pharmaceutical company.
Target indication analysis of market including competition and potential. Clinical development plan cdp part of your strategic development plan. Team minutes template issues boxed things that will cause delay cost overrun or that may impact a. April 27 2013 by marcio barra.
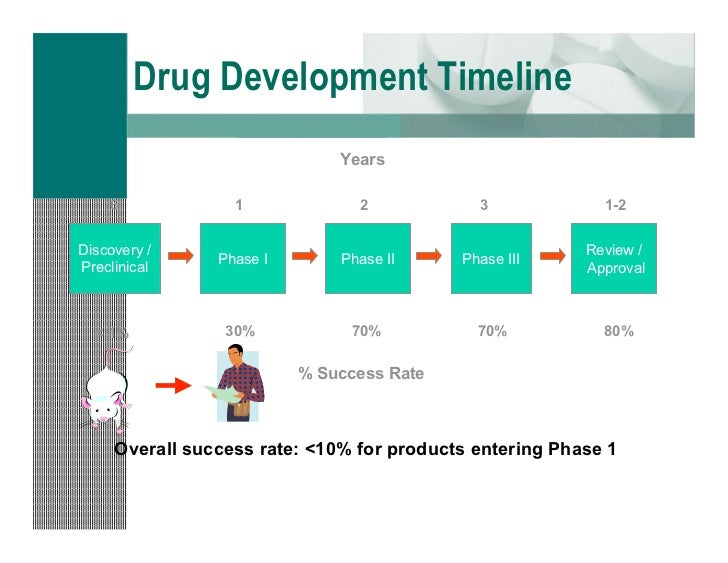
Predicting the probability of developing a successful cancer therapy tony sabin csg feb 2010 contents brief overview of clinical drug development process aims of clinical development stages of development cost decision making in the pharmaceutical industry historical perspective bayesian model for predicting success in pancreatic cancer first in human trials single centre healthy volunteer. Clinical development plan cdp a well thought through clearly documented and well structured cdp is the foundation of a good clinical development and an essential document when registering the intent to develop a new medicine or therapy. Please note that this page has been updated for 2015 following a quality check and review of the templates and many new ones have been added. Welcome to global health trials tools and templates library.
The second is the clinical development plan cdp which can be viewed as the map of how to get there. Sufficient time and detail should be spent on the clinical program. Robustness of the clinical data establish the pms and ci plan monitor common specifications and new guidances compliance with mdd 9342ec iteration of medical devices new product introduction clinical roadmap shall be established as early as possible in the product development establish whether a ci shall be. Review the concept of clinical development discuss the components of the clinical.
Detailed evaluation of target indications including unmet needs. The clinical development plan. A global development plan is for internal use only and should not be shared with fda only in the broadest terms or other regulatory body.