Clinical Trial Monitoring Plan Template

Is a document that outlines the principles of risk based monitoring and may assist you in the customization of your monitoring plan.
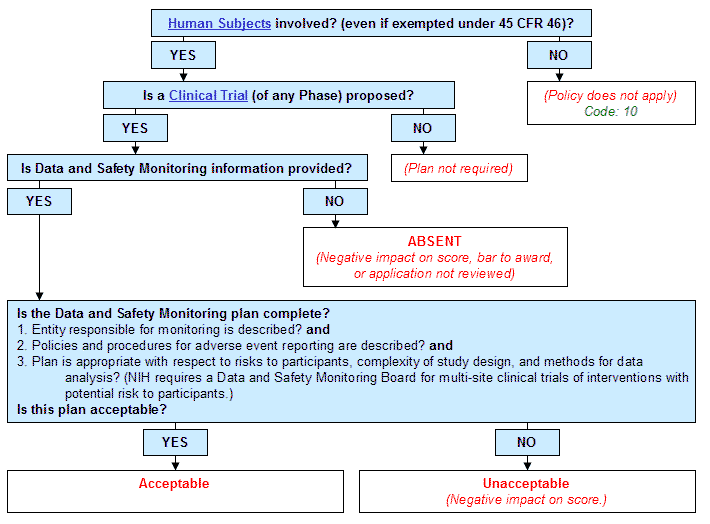
Clinical trial monitoring plan template. Risk based monitoring plan rbmp describes the rbm strategy and. The monitoring risk category for this study is insert. The sponsor risk assessment form and the trial risk based monitoring strategy appendices 2 3 to sop s 1007 will facilitate the development of the monitoring plan. The purpose of trial monitoring is to verify the following.
The goal of the dsmp is to provide a general description of a plan that. Investigators should consider using this template when developing the data and safety monitoring plan dsmp for clinical studies supported by the national institute of arthritis and musculoskeletal and skin diseases niams. Welcome to global health trials tools and templates library. With the finalization of ich gcp r2 sponsors and cros are actively looking into implementation of a risk based monitoring rbm approach to their clinical trials to achieve the objectives related to enhanced data quality better monitoring of patient safety and creating efficiencies in overall operations.
The monitoring of a trial is one of the key activities undertaken as part of the trials management. The type of monitoring undertaken either onsite remote or central and the frequency and. The mhra accepts a risk adapted approach to trial management and the advice specific to trial monitoring can be found in appendix 2 of the risk adapted approaches to the management of clinical trials of investigational medicinal products. All clinical trials require study specific monitoring procedures to ensure participant safety and data integrity.
Setting up the structure for a systematic approach clinical consulting. The toolbox contains templates sample forms and information materials to assist clinical investigators in the development and conduct of high quality clinical research studies. Data and safety monitoring plan dsmp template and guidelines ms word 37k and dsmp checklist ms word 43k were developed to assist investigators in preparation of a sound data and safety monitoring plan. Monitoring is defined as the act of overseeing the progress of a clinical trial and of ensuring that it is conducted recorded and reported in accordance with the protocol sops the principles of gcp and the medicines for human use clinical trails regulations where applicable.
Paper on risk based quality management in clinical trials3 namsas rbm. Please note that this page has been updated for 2015 following a quality check and review of the templates and many new ones have been added.