Clinical Trial Site Activation Letter Template

Planning start up.
Clinical trial site activation letter template. Please ensure that you read and adapt them carefully for your own setting and that you reference global health trials and the global health network when you use them. Informed consent template for clinical trials. Page 2 of 2. Risk assessment template.
Confirm ready to activate. Nih fda phase 2 and 3 indide clinical trial protocol template. The program official will verify that ctoa have been met and will approve the site for activation by signing the checklist. Confirm ready to activate with sponsor.
To improve start up times and outcomes one needs an experienced clinical research investigator motivated and capable team members and efficient. Site readiness checklist for vaccine trial. Welcome to global health trials tools and templates library. Page 1 of 2.
Site activation is the driver in patient enrollment many in the industry believe that patient enrollment is the responsibility of the investigator sites and sponsors play only a supporting role in the processthis is only part of the truth. Budget 700u state required financial form only use if private sponsor form 800 federal form required for all human subjects studies private sponsor or otherwise exhibit b university budget template most of these forms can be found at the sponsored programs website. After your visit you will likely need to complete a report template or assessment and send a follow up letter to thank the site for hosting you and inform them whether or not they have been chosen to participate in the study. Upon receipt of this notice the site may commence the study.
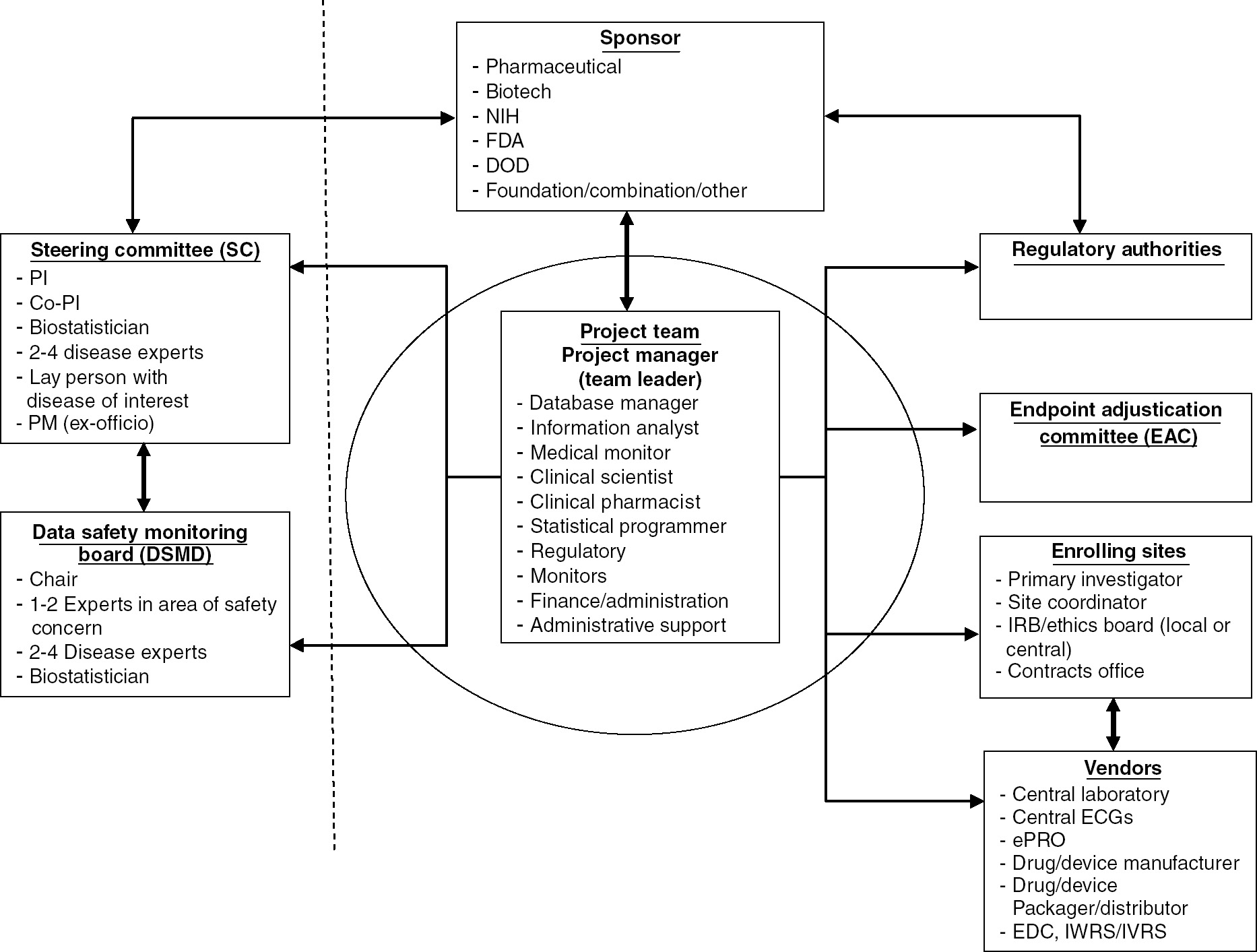
Clinical trial site activation and efficient study start up for both site based and remotede centralized trials are critical to drug development programs in terms of time cost and quality of data. Toolkit interventional studies. The templates below have been shared by other groups and are free to use and adapt for your research studies. Start up checklist clinical team.
9 contract budget contract also known as cta for a clinical trial agreement. This is typically a 2 4 hour visit. For clinical trials a site specific study activation notice will be issued by noclor. Skcc clinical research organization.
Chart 1 shows the relationship between investigator site activation cycle time and patient. No study procedures should be undertaken before the activation notice is received. Ethics committee approval letter template. Once all study activation requirements have been met the study can commence.
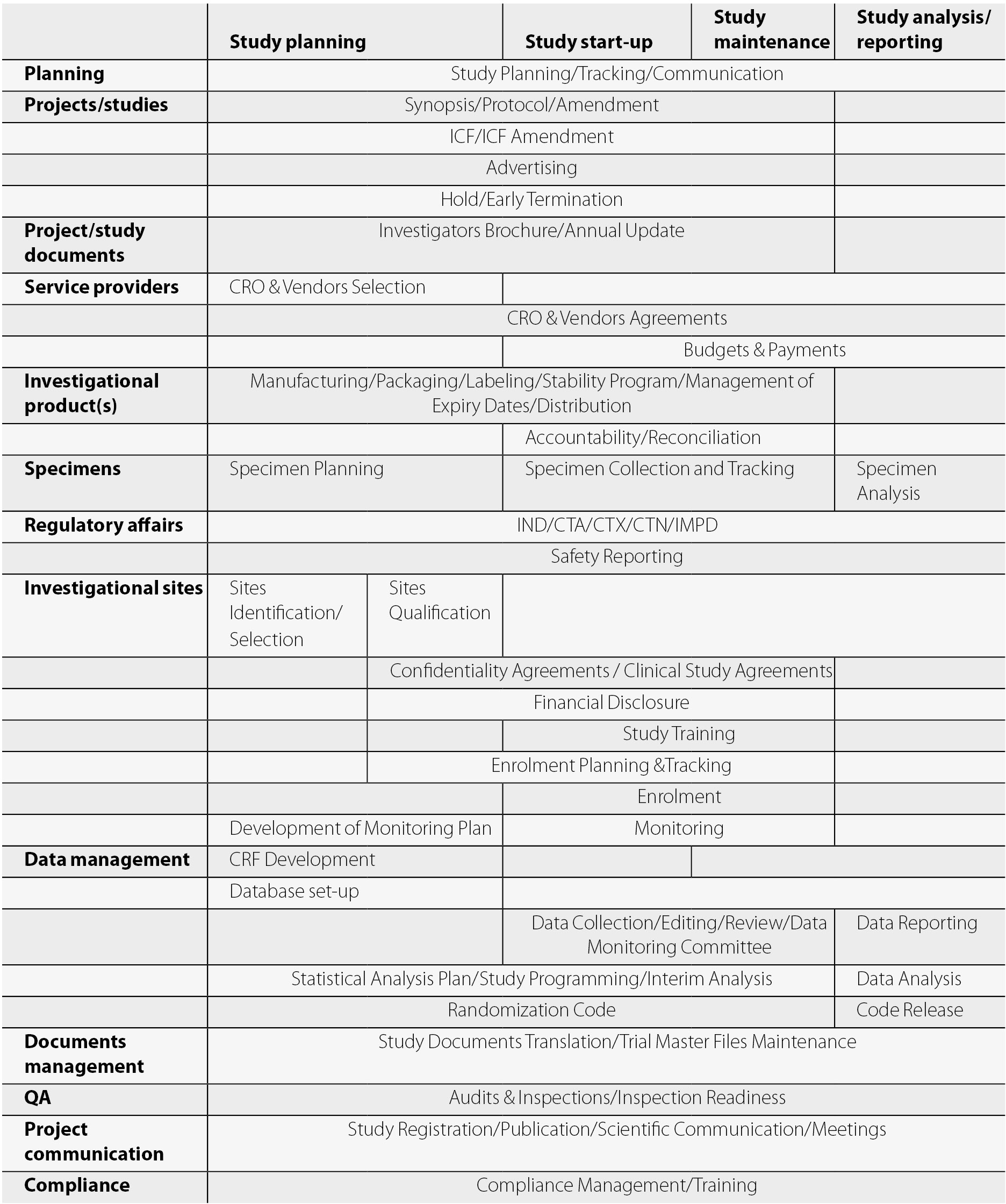
Provides a list of items required prior to site activation and tracks completion of tasks. Site initiation activation and close out sop.