Data Management Plan Template Clinical Trial

Should be used to develop a data management plan dmp to accompany a research proposal.
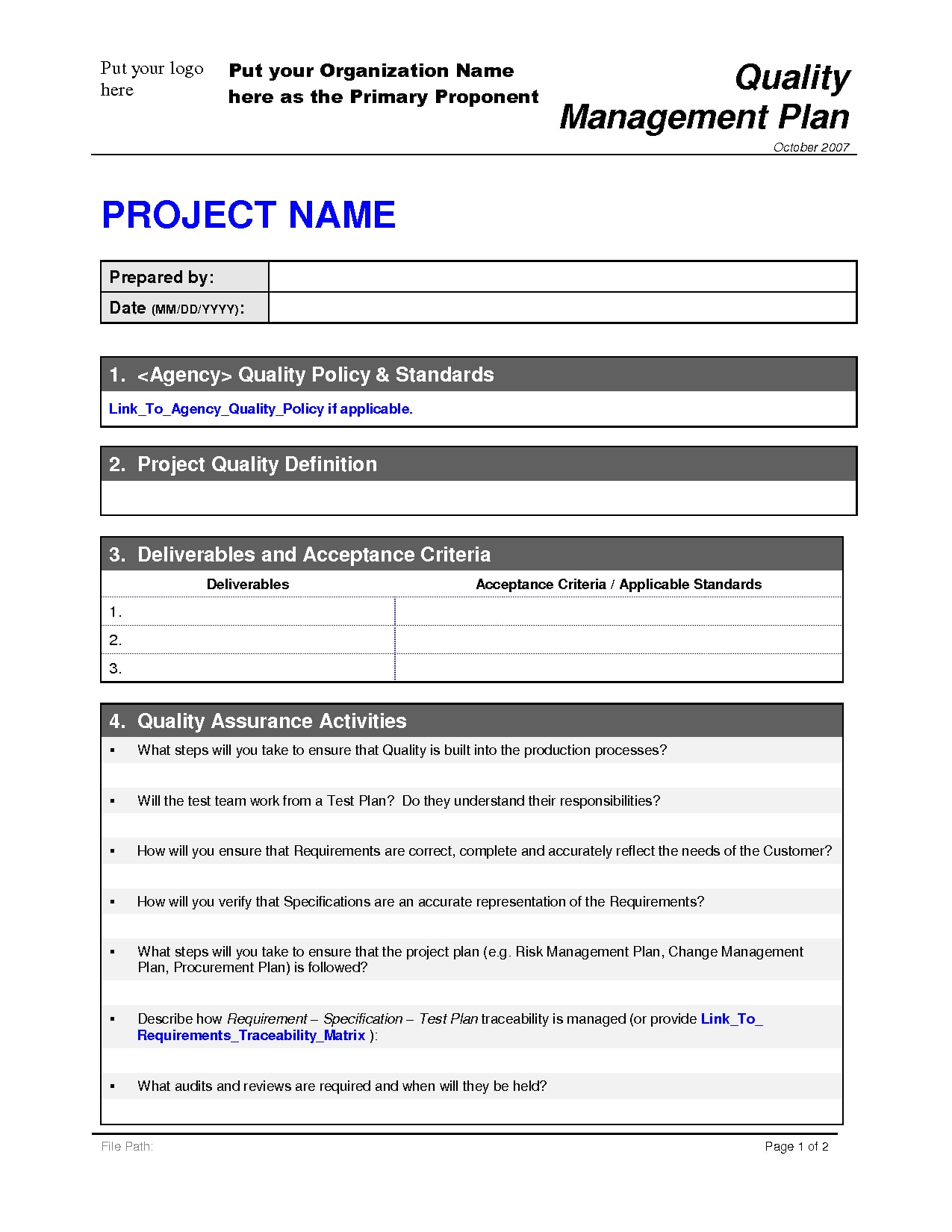
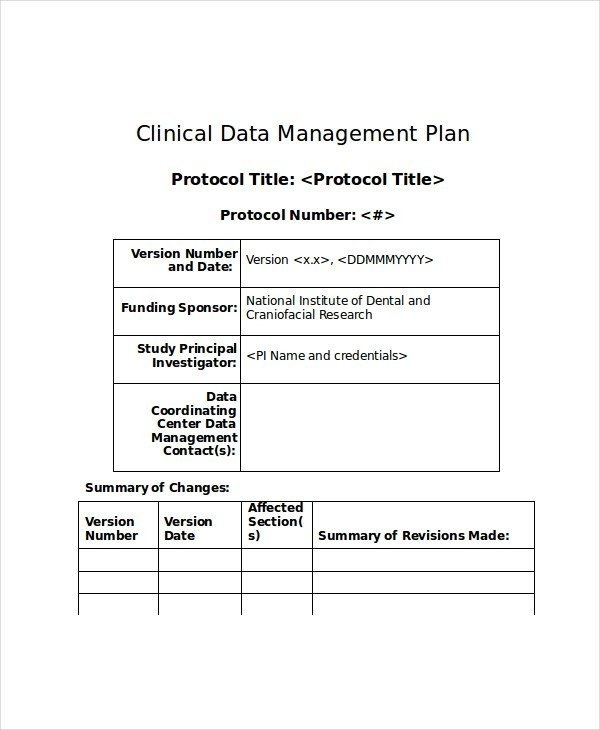
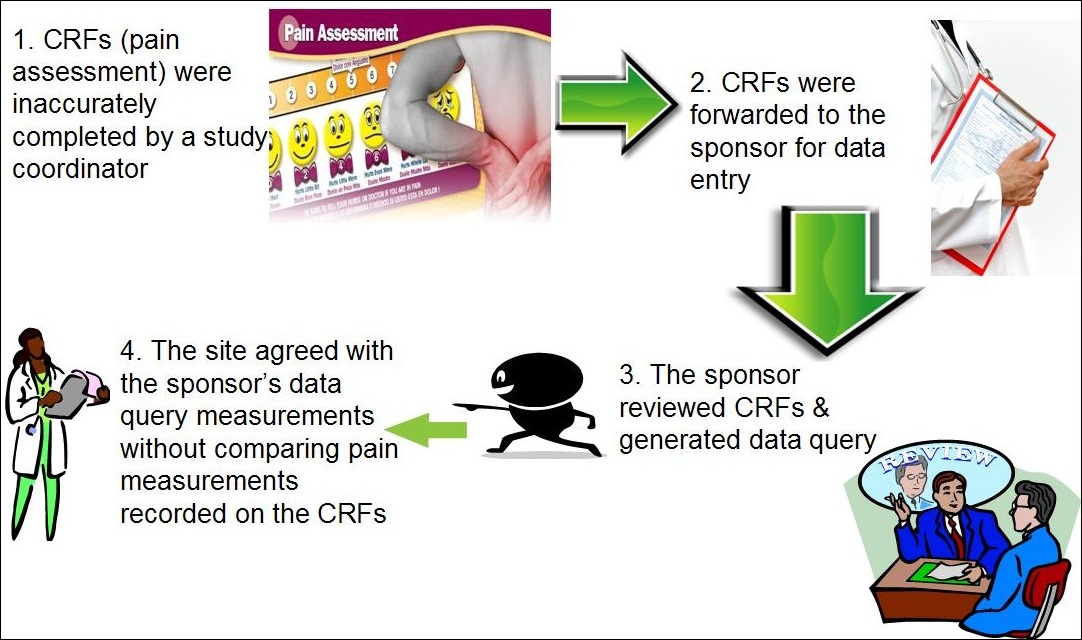
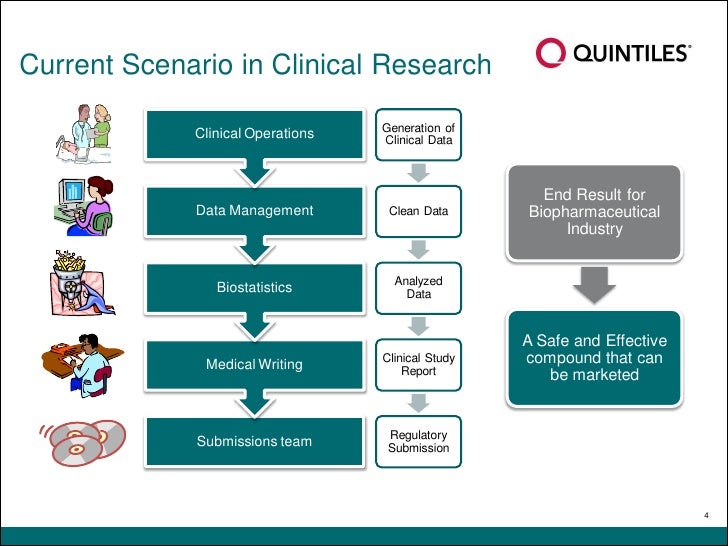
Data management plan template clinical trial. Essential documents checklist investigator site file master file set up and maintenance sop. This clinical data management plan cdmp template may be employed for studies using an electronic data capture system edc unless another template has been agreed upon. The purpose of the data management plan dmp is to assure that sites have procedures and controls in place to ensure protection of human subjects participating in a study and the authenticity integrity and confidentiality of study data. The notes in italics provide further context and guidance for its completion.
Planning for the trial and data management. The mrc htmr network host a series of webinars on trial conduct including monitoring trials efficiently. The role of central statistical monitoring. Protocol template a protocol is a document that describes the background rationale objectives design methodology statistical considerations and organization of a trial.
Template for a data management plan. Clinical trial agreement cta with sponsors or contract research organisations cros sop. Serious adverse events form template. Agreements approvals and contracts.
Before you begin the clinical trial. Many clinical research professionals recommend including patients in the planning phase of clinical trials at least as stakeholders to review the plan. Clinical study report template. Where substantial data is generated from the research the dmp will be more in depth and therefore likely to be 2 or 3 pages long.
Archiving trial data sop. Archival of essential documents sop. Separate files will be managed for the two kinds. Data safety monitoring board dsmb charter.
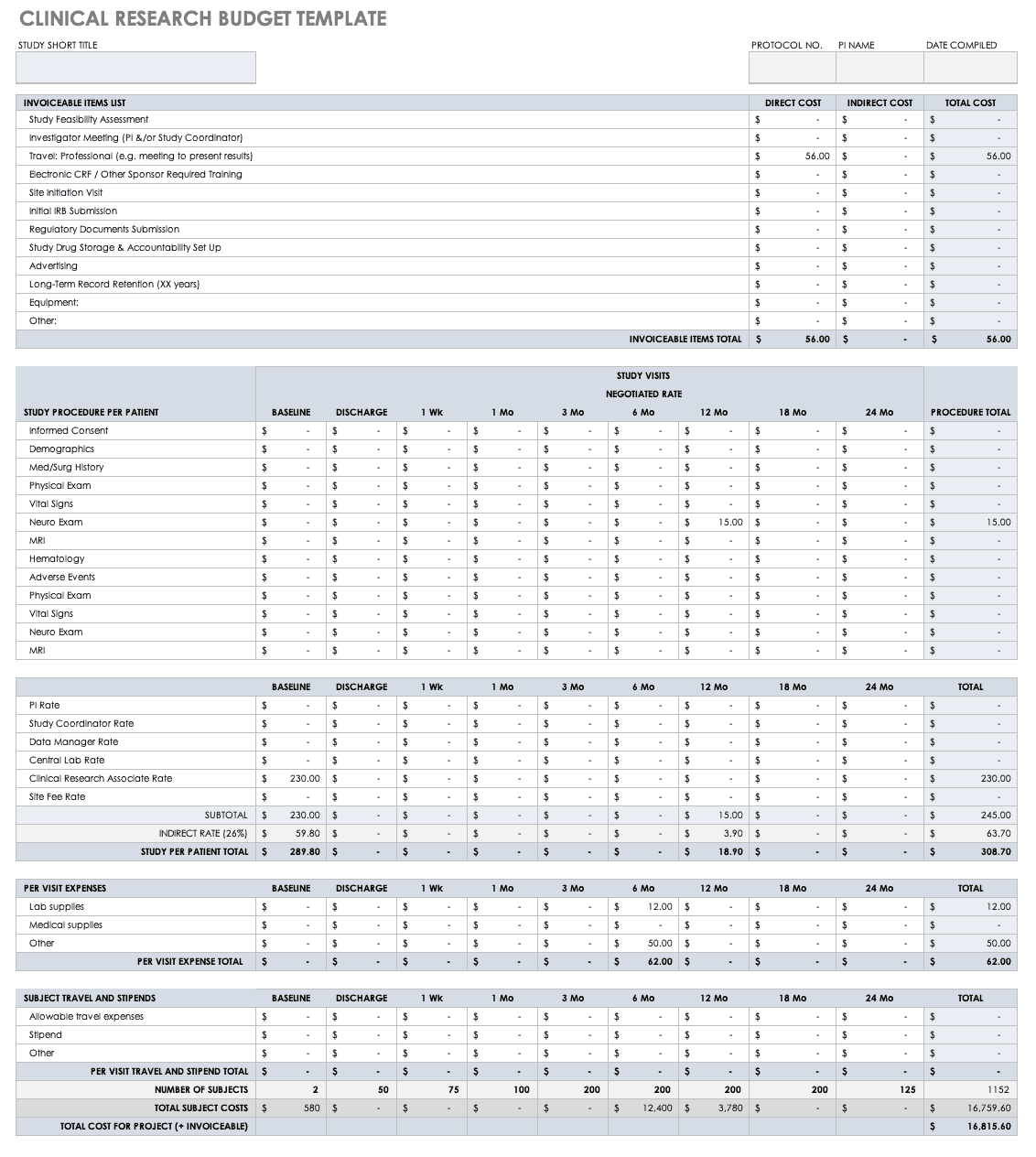
By completing the plan early and allowing potential participants to review it you help improve recruitment and retention during the trial. The trial managers network tmn is a source of practical support and guidance on the trial management process and has published comprehensive guidance. Confidentiality and nda template. Budget monitoring tool with example data.
Budget monitoring tool. The ich good clinical practice guidelines specify some topics that should generally be included in a protocol. Examples and templates for developing and conducting a clinical trial in a pbrn. Interactions with iec institutional ethics committee sop.
Clinical trial agreement log. Home clinical innovations multisite clinical trials in pbrn toolkit examples and templates. Data will be stored in a cvs system and checked in and out for purposes of versioning. A guide to efficient trial management.