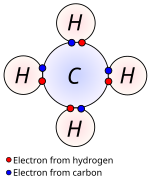
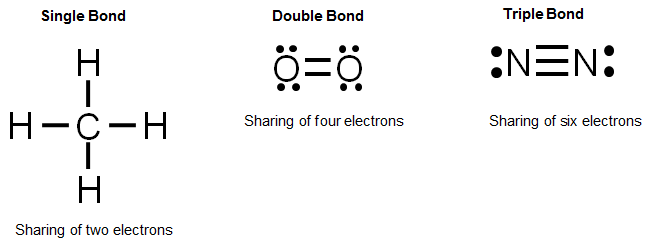
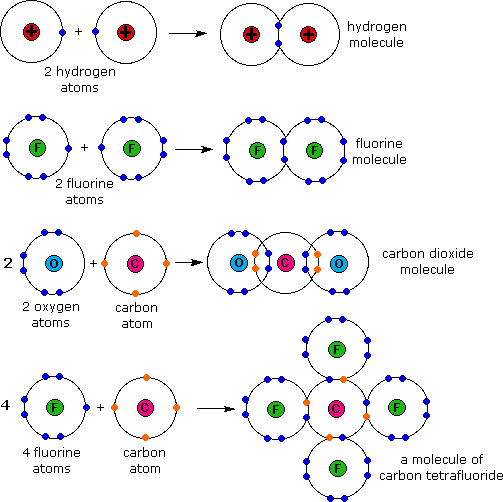
Diagram Of Chemical Bonding

They used the concept of inertness of noble gases to explain the fundamentals of chemical bonding.
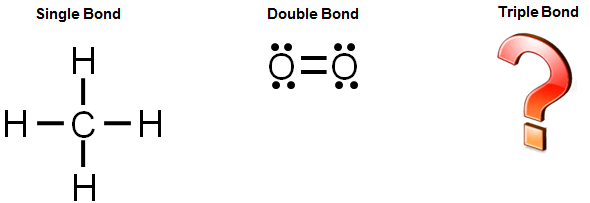
Diagram of chemical bonding. A dot cross diagram consists of nothing but circles dots and crosses. We will learn about the different kinds of bonds ways chemists draw bonds and molecules and how the type of chemical bonding affects the bulk properties of a material. There are three types of strong chemical bonds. O 2 triple bond.
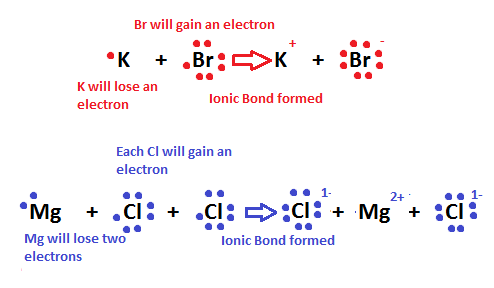
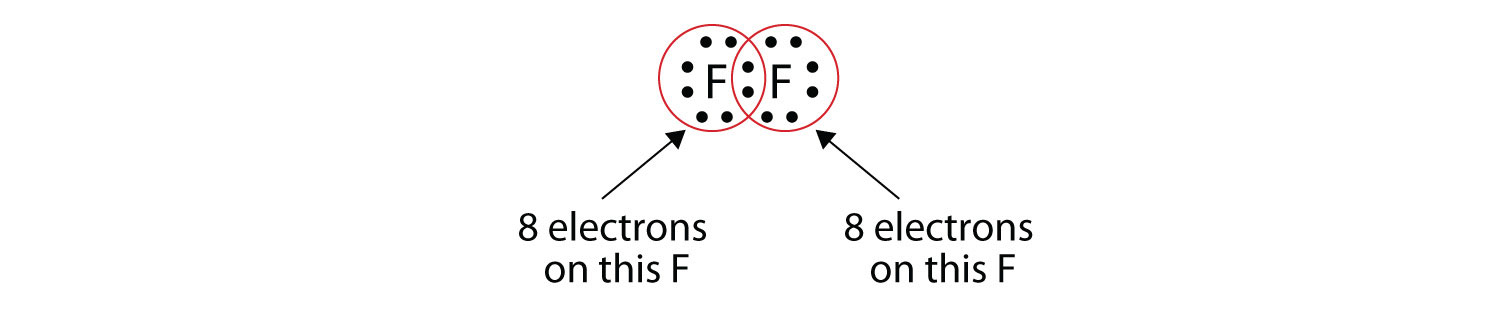
Type of chemical bond that involves the transfer of electrons between atoms is an bond. The dot represents electrons from one atom while the cross represents electrons from the other atom. Kossel and lewis were the first scientists to explain the formation of chemical bonds successfully. The chemical bonding within the molecule is also shown either explicitly or implicitly.
Ionic fluorine f and bromine br are in the same group on the periodic table. Chemical bonds are the glue that hold molecules together. When one pair of the electron is shared it means one covalent bond is formed. The structural formula of a chemical compound is a graphic representation of the molecular structure showing how the atoms are possibly arranged in the real three dimensional space.
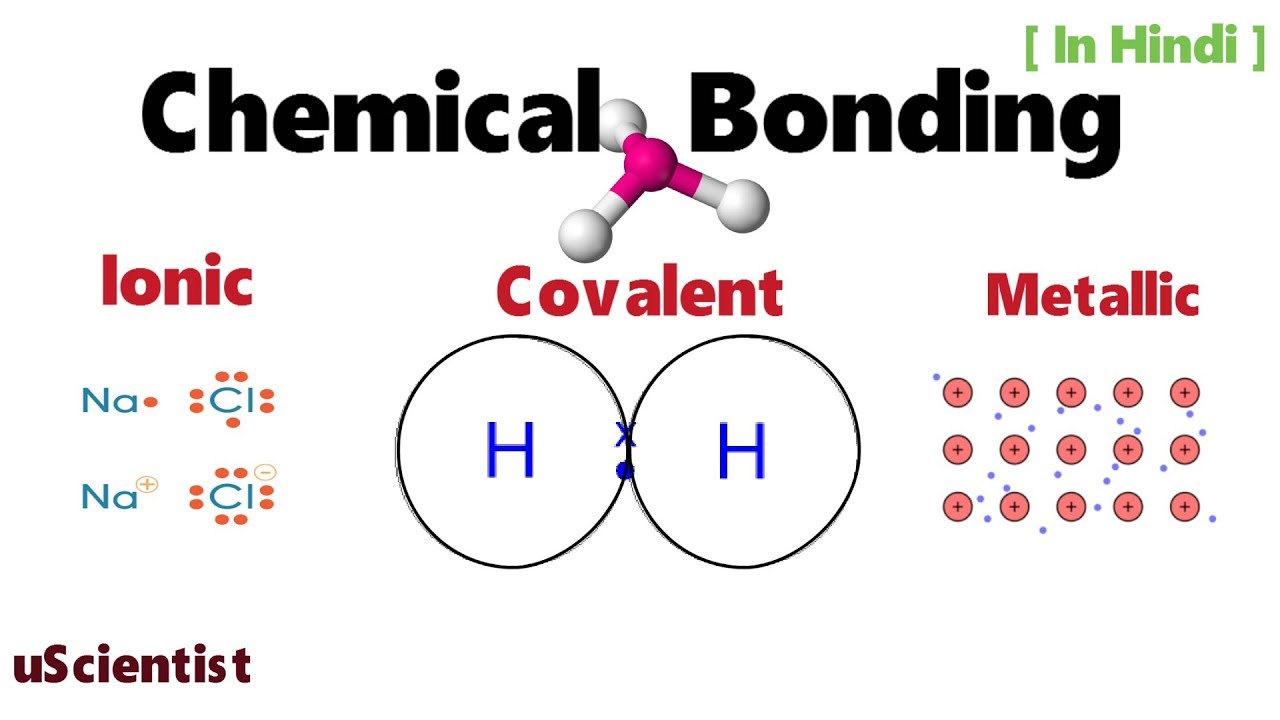
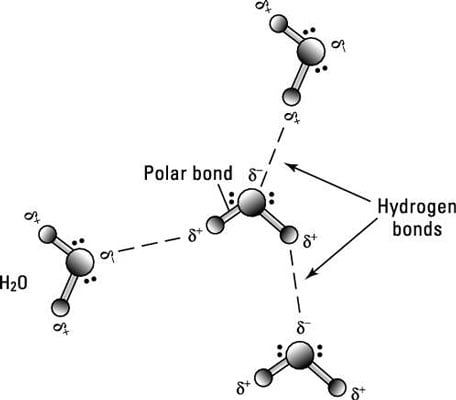
In covalent bonding the particles are atoms usually both non metals share pairs of electrons to form the bond. Because opposite charges attract the atoms bond together to form a molecule. We will cover electronegativity lewis dot structures vsepr bond hybridization and ionic covalent and metallic bonds. The dot cross diagram is an essential part of explaining how covalent bonding works.
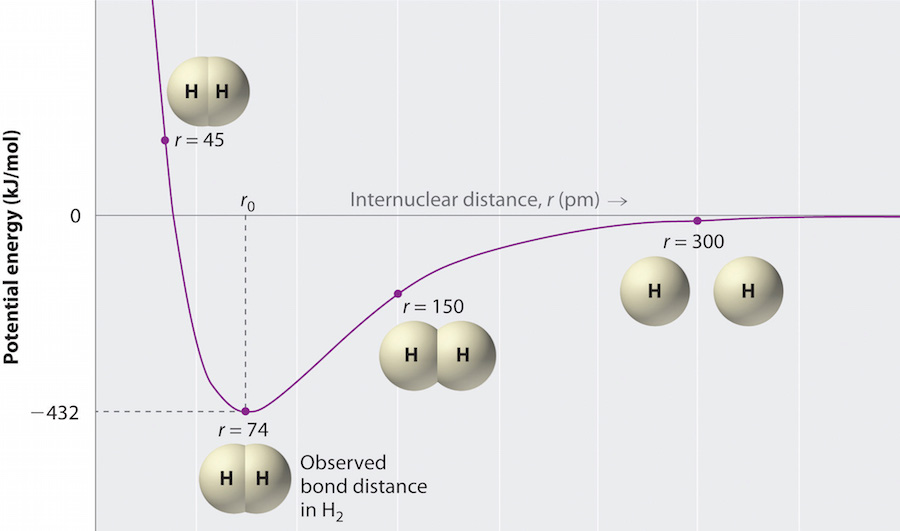
When atoms approach one another their nuclei and electrons interact and tend to distribute themselves in space in such a way that the total energy is lower than it would be in any alternative arrangement. Involves two electron pairs eg. Unlike chemical formulas which have a limited number of symbols and are capable of only limited descriptive power structural formulas provide a more complete geometric representation of the molecular structure. Ionic bonding electrons are transferred between valence shells of atoms ionic compounds are made of ions ionic compounds are called salts or crystals not molecules ionic bonding always formed between metals and non metals metals non metals lost e gained e ionic bonding electronegativity difference 20 look up e neg of the atoms in the bond and subtract nacl cacl2 compounds with polyatomic ions nano3 anion cation hard solid at 22oc high mp temperatures nonconductors of.
One of the resulting ions carries a negative charge anion and the other ion carries a positive charge cation. In ionic bonding the particles atoms or a group of atoms form oppositely charged ions. Ionic bonding involves a transfer of an electron so one atom gains an electron while one atom loses an electron. According to their theory of ionic and chemical bonding ionic bonding involves the transfer of electrons between atoms.
Ionic covalent and metallic. Involves an electron pair eg. Chemical bonds attractive force holding atoms together single bond.