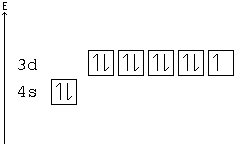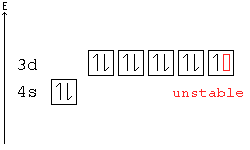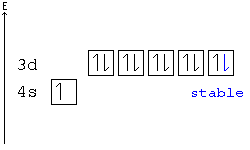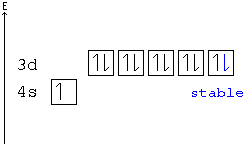Electron Configuration Orbital Diagram For Nickel

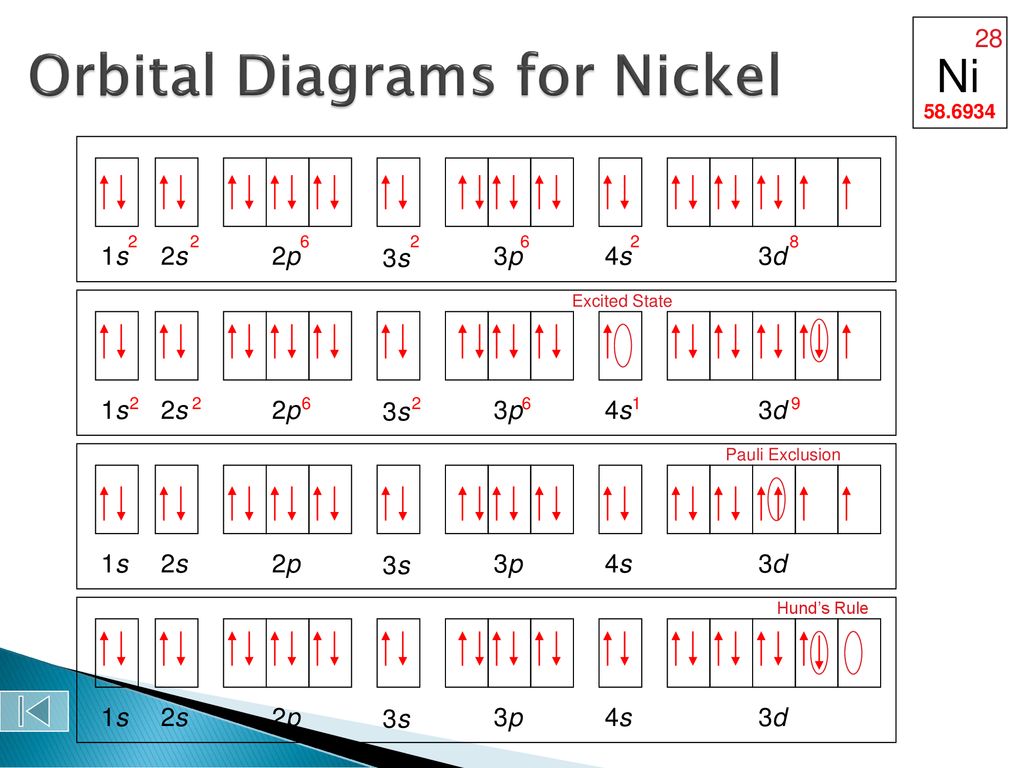
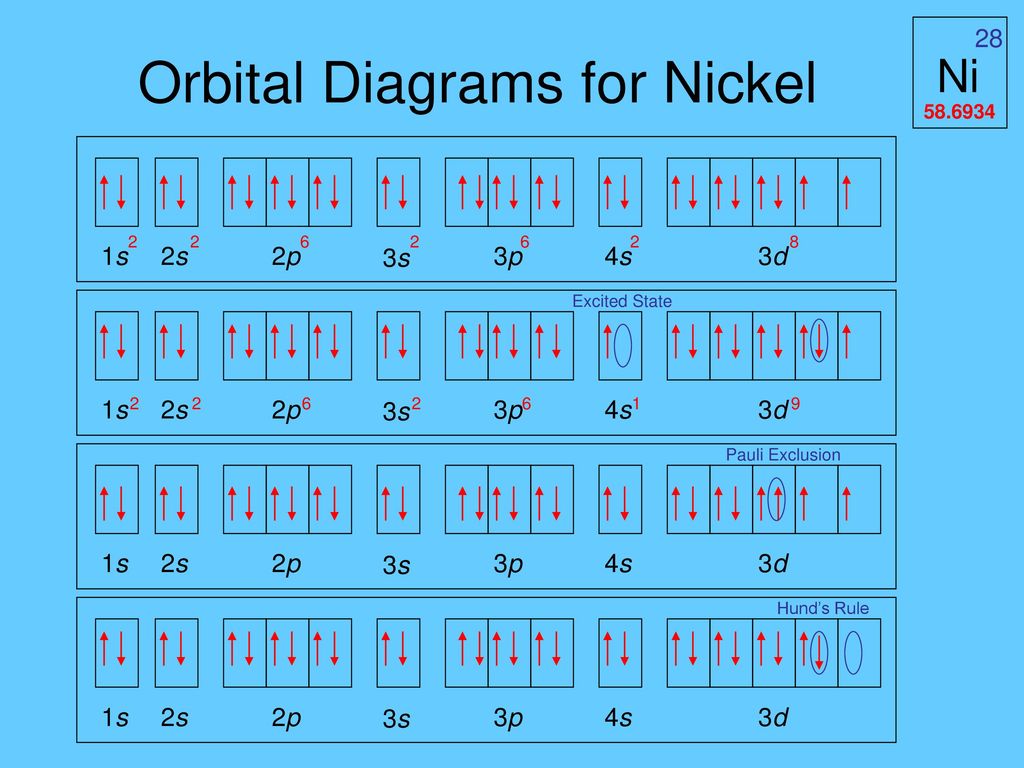
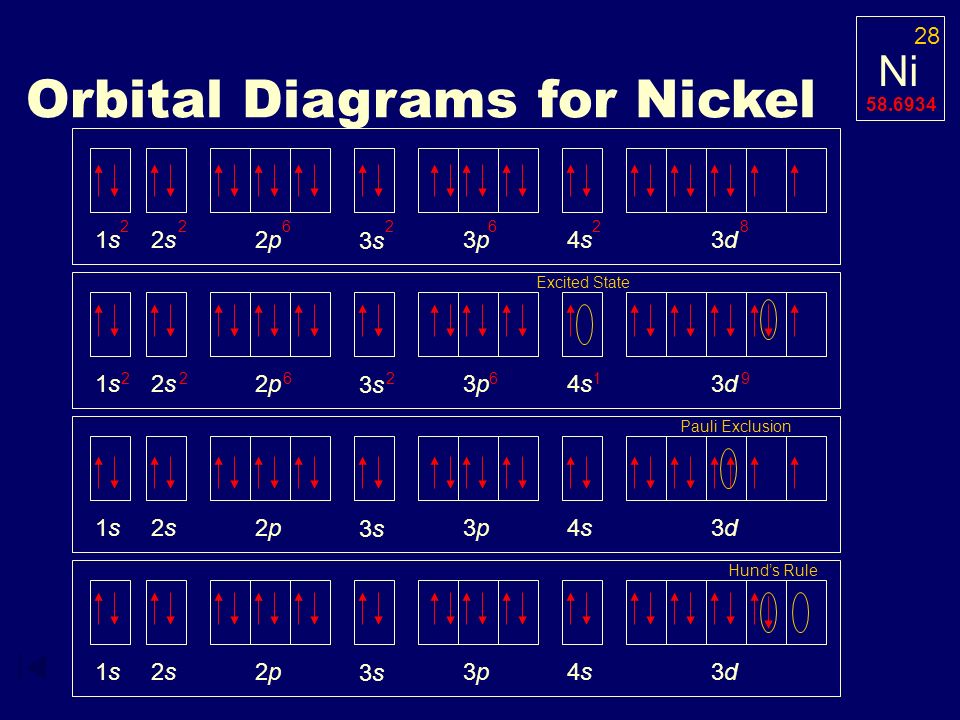
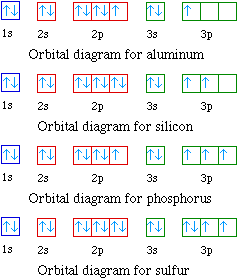
In all of the cases both up and down arrows are filled with the exception of the 3d shell where the last two are up.
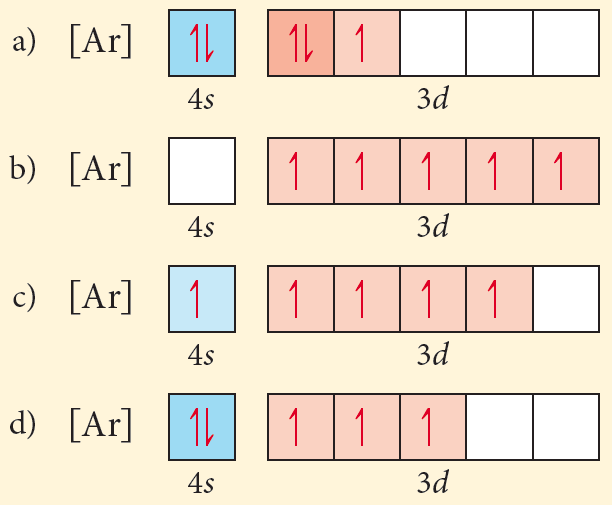
Electron configuration orbital diagram for nickel. How many unshared pairs of electrons are in this orbital diagram. In addition to listing the principle quantum number n and the subshell ell the orbital diagram shows all the different orientations and the spin of every electron. Click to see image answer choices. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 8 4s 2.
The orbital diagram for nickel is as follows. Electron configuration is a way to draw an orbital diagram for a late electron. The electron configuration of ni2 is 1s22s22p63s23p63d8. Phosphorus 1s 2s 2p 3s 3p 4s 3d 4p.
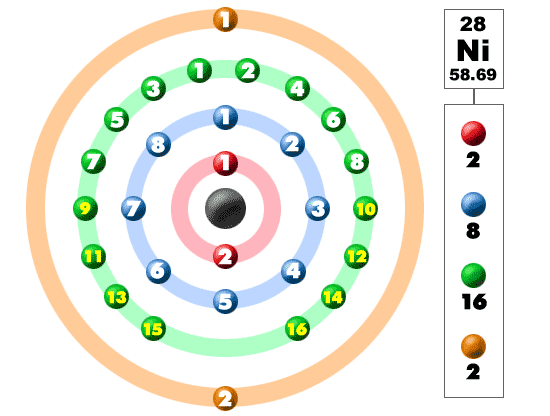
What atom matches this electron configuration. 1s2 2s2 2p6 3s2 3p6 4s2 3d8. Nickel ni has an atomic mass of 28. For this type of diagram the core electrons are.
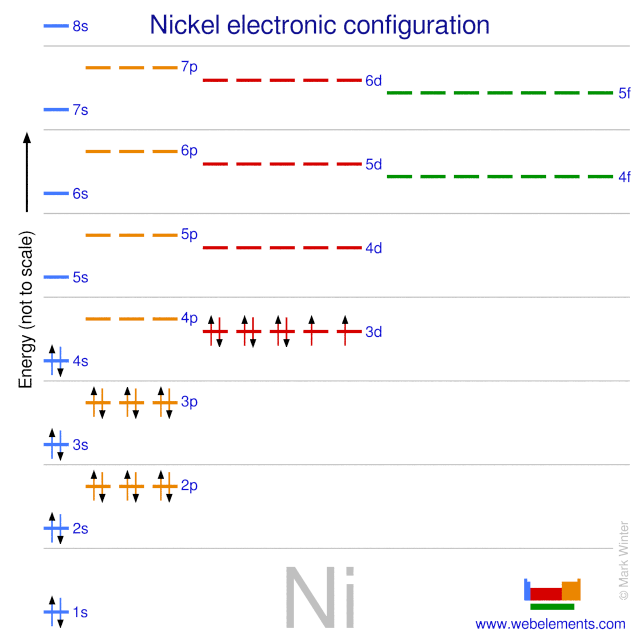
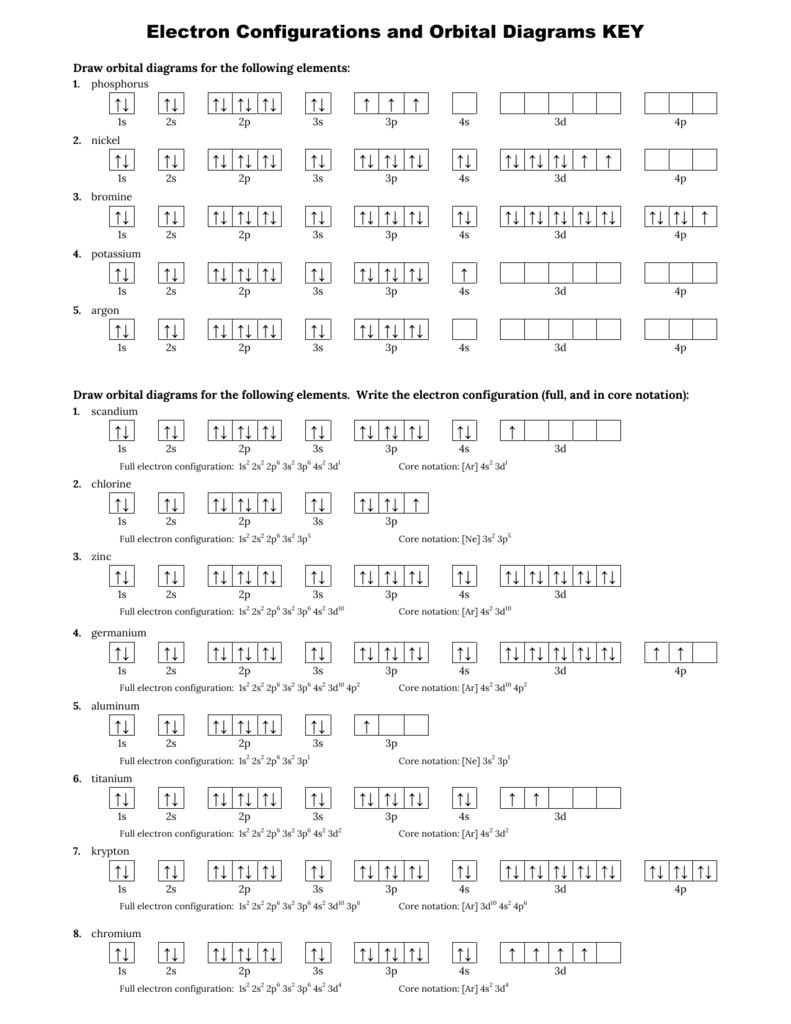
Ar 4s2 3d8 or 1s2 2s2 2p6 3s2 3p6 4s2 3d8. Key introduces another representation of electron configurations using orbital box diagrams to explain bonding as well as providing an explanation as to how multivalency can occur. Find out about its chemical and physical properties states energy electrons oxidation and more. Electron configurations and orbital diagrams key draw orbital diagrams for the following elements.
How many orbitals are in the 4d sublevel. Electron configuration ar 3d 8 4s 2. Nickel is atomic number 28. 1s 2 2s 2 2p 6 3s 2 preview this quiz on quizizz.
Only 2 electrons per orbital they must have opposite. The filling rules are as follows.