Eu Gmp Certificate

Eudralex volume 4 good manufacturing practice gmp guidelines volume 4 of the rules governing medicinal products in the european union contains guidance for the interpretation of the principles and guidelines of good manufacturing practices for medicinal products for human and veterinary use laid down in commission directives 91356eec.
Eu gmp certificate. If a special need arises from authorities in non eu countries. This content applies to human and veterinary medicines. The eudragmdp database is maintained and operated by the ema. The european medicines agency ema coordinates inspections to verify compliance with these standards and plays a key role in harmonising gmp activities at european union eu level.
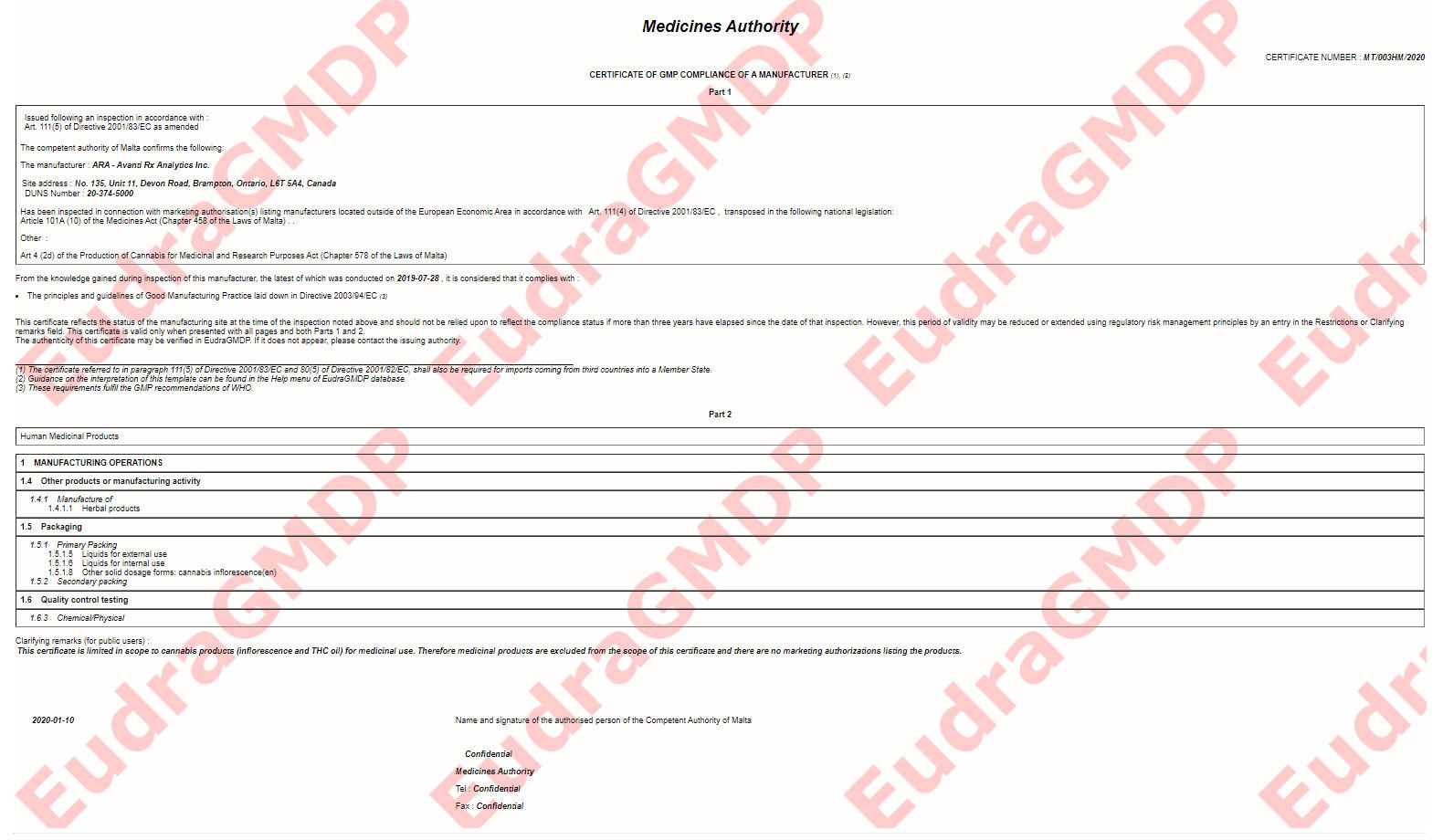
The requirement for the written confirmation can only be waived if the third country is included by the european commission after assessment in a list of countries with an equivalent system of supervision and inspection or exceptionally in order to ensure availability of medicines in the eu market if a gmp certificate for the site has been. We only issue who gmp certificates statement of gmp compliance in extraordinary cases eg. Microsoft word proposal for eu gmp certificate finaldoc. Certificate of gmp compliance of a manufacturer part 1.
Ema is not responsible for the contents of the database. Guidance on the certification by a qualified person qp and on batch release within the european union eu of medicinal products gmp search engine search in gmp database training conference guidelines news press conference folders. The european medicines agencys ema provides answers to frequently asked questions on good manufacturing practice gmp and good distribution practice gdp as discussed and agreed by the gmpgdp inspectors working group. The who gmp certificate.
Any questions on its content should be addressed to the relevant national competent authority. At the request of the medicinal products manufacturer a competent eu authority can issue a who gmp certificate for a company that owns a manufacturing authorisation and therefore is monitored according to gmp. Access to the general public is granted in order to enhance availability of information related to the ema mandate. Compilation of community procedures.
Good manufacturing practice gmp describes the minimum standard that a medicines manufacturer must meet in their production processes. We recommend that you use the companys gmp certificate in the ema format instead of requesting who gmp certificates. Gmp certification and gmp certificate in europe an overview.

.jpg)















