Fda Certification Check

With this latest approval and on september 1 2008 the stx joins the fda and the states of illinois iowa and south carolina as certifying agencies.
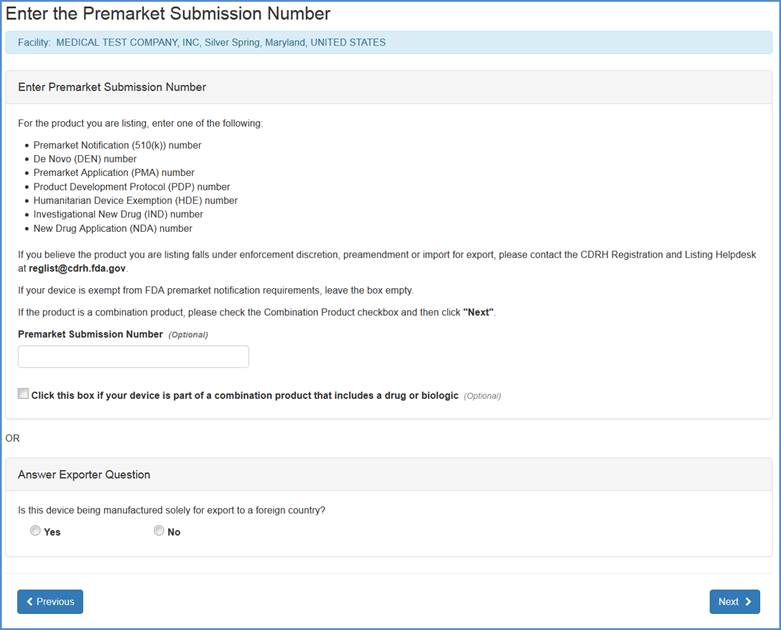
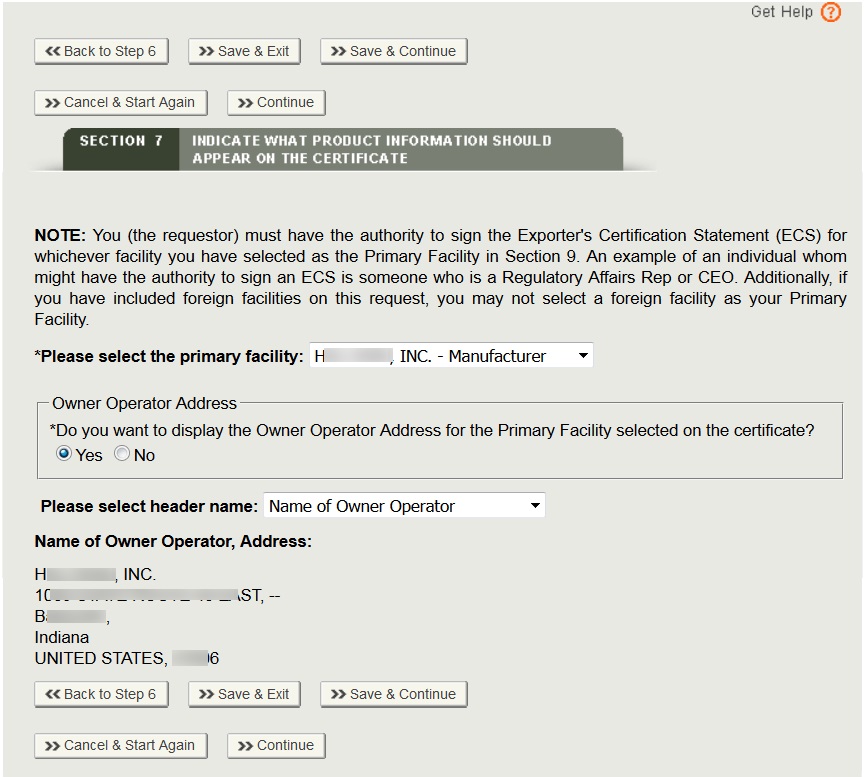
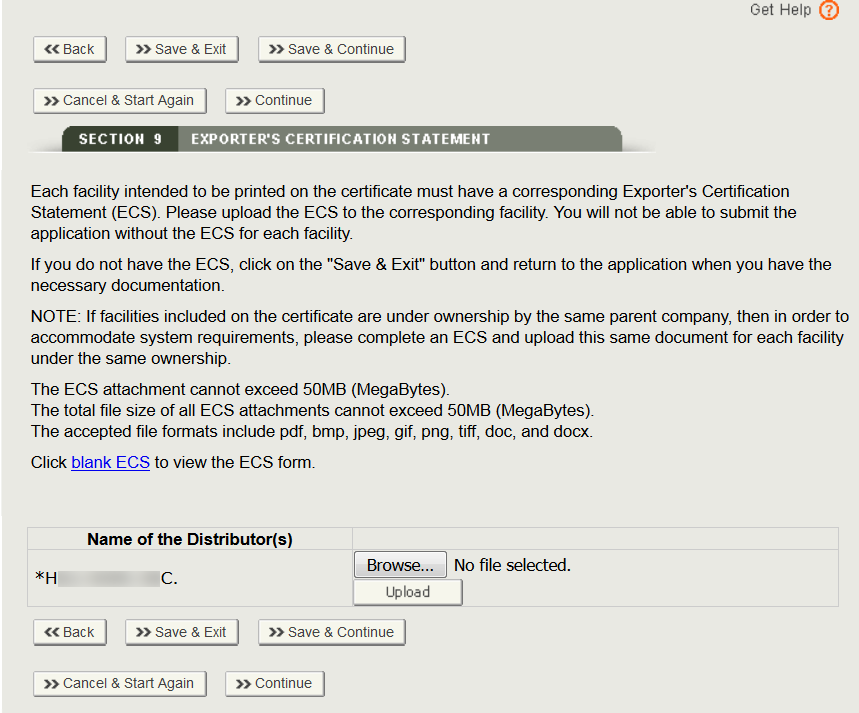
Fda certification check. As part of these services dun bradstreet may look to verify information such as. Fda regulations require all blood establishments that collect manufacture prepare store under controlled conditions for further distribution or process blood and blood products to register. If you have any questions please contact the mqsa facility hotline at 1 800 838 7715. This guidance document is intended to provide a general description of fda export certificates to industry and foreign governments.
The certification status of facilities may change so fda suggests that you check the facilitys current status and look for the mqsa certificate. Any representation of fda registration number on product label or labeling which implies fda certification or fda approval of a facility or product is misleading and may cause misbranding of the product. All compliance actions for any stx mqsa inspection findings. The food and drug administration fda conducts careful inspections of regulated facilities to determine a firms compliance with regulations and the food drug and cosmetic act.
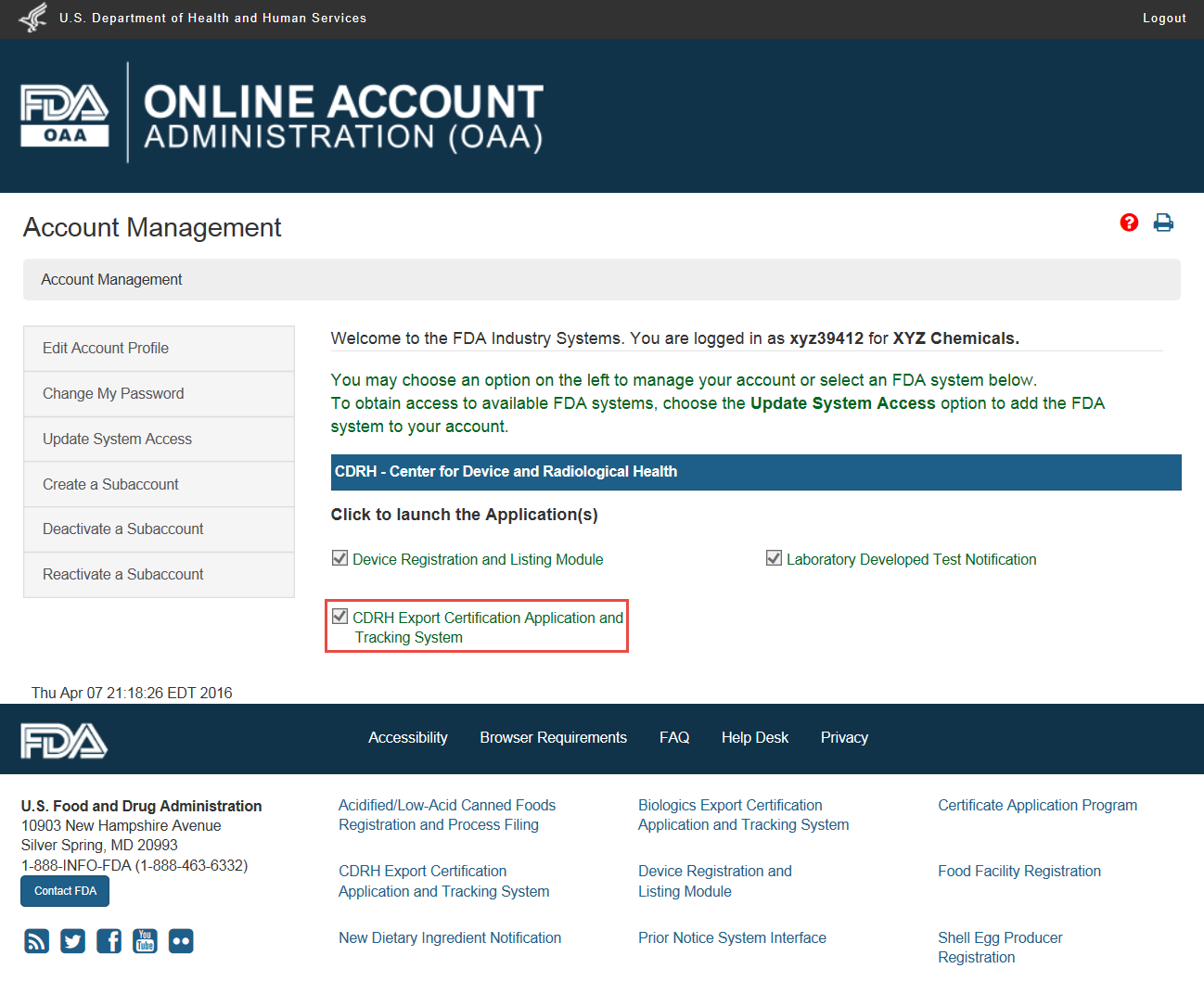
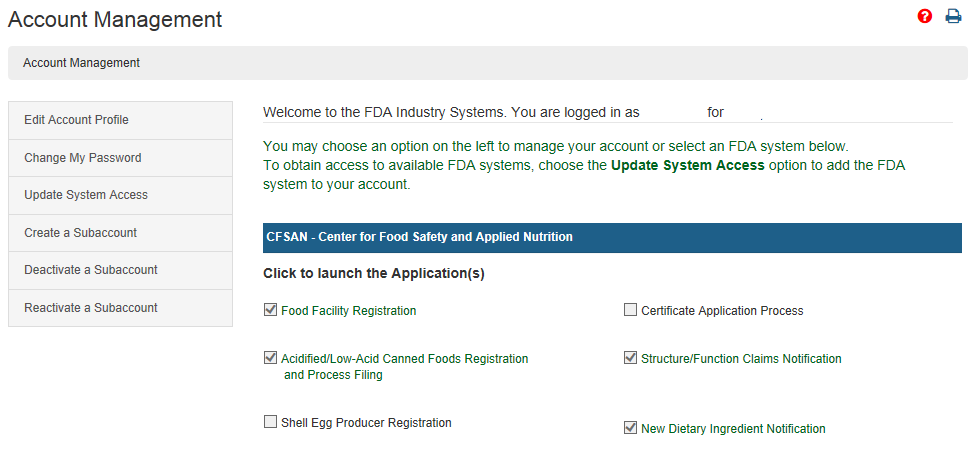
Firms exporting products from the united states are often asked by foreign customers or foreign governments to supply a certification relating to products subject to the federal food drug.