Gmp Auditor Certification
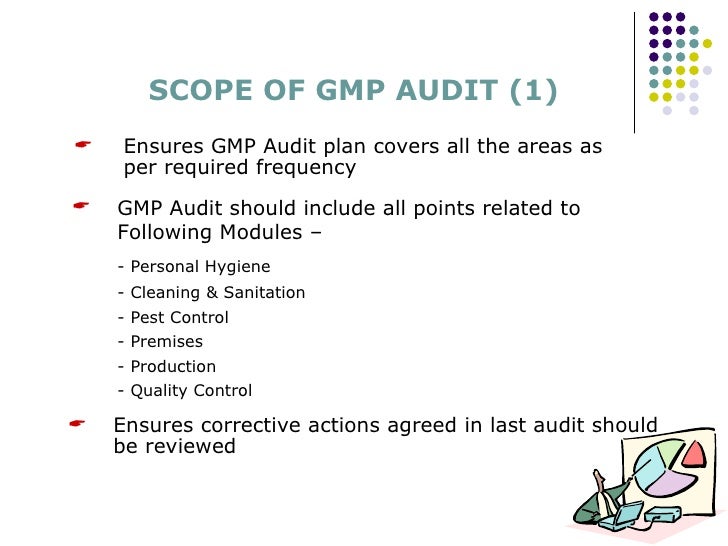
A continuous training in all gmp related areas is therefore essential for all gmp auditors.
Gmp auditor certification. Gmp registration services in new york specifies the requirement on hiring and training those employees within your manufacturing unit who can at least understand your process and document it so that it can be demonstrated to the auditors who come from the certification body in order to audit your system to certify on this international. Find out more about nsfs pharmaceutical auditor training and audit support services. This course teaches you the purpose of a pharmaceutical quality management system of pharmaceutical quality systems standards of management system audits in the supply chain. Watch a video to learn more about our cqi and irca certified auditor training program.
Download the quality auditor certification fact sheet pdf 61 kb. This 3 day course is aimed at quality assurance auditors and production management for level 2 internal audits and supplier auditing. This auditing for gmp course is specifically designed to address the challenges of gmp auditing for the pharmaceutical industry and present the basic competencies required to effectively perform the auditors assigned responsibilities and contribute to the improvement of auditor performance within a regulated industry. Pharmaceutical gmp auditor training.
Recognising this need for further professional knowledge development the eca academy has set up a programme for initial and continuous qualification. Assurance of the safety and quality of food is an important consideration for consumers today. Learn prepare apply certify recertify. The certified quality auditor analyzes all elements of a quality system and judges its degree of adherence to the criteria of industrial management and quality evaluation and control systems.
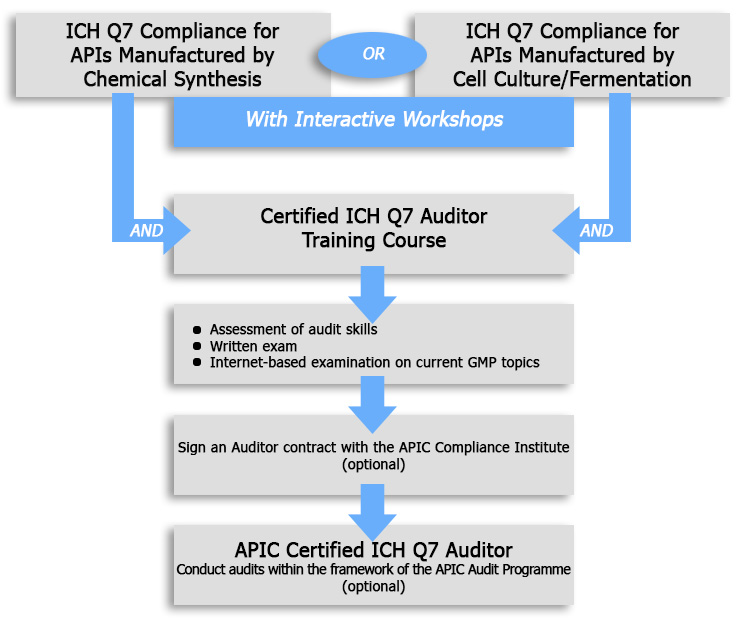
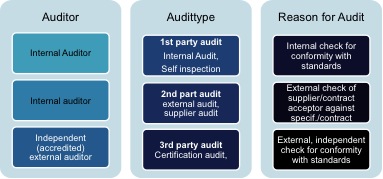
The course trains auditors how to professionally plan perform report and follow up internal and supplier audits and is set in a pharmaceutical context throughout the whole course. The certified pharmaceutical gmp professional understands the good manufacturing practices gmp as regulated and guided by national and international agencies for the pharmaceutical industry. Current gmp good manufacturing practice legislation requires that there are internal and external audit programmes operating as part of an integrated quality system. Here an additional certificate can be obtained eca certified gmp auditor.
The pharmaceutical gmp auditorlead auditor course covers the requirements of european union gmp eu gmp including the requirements of ich q9 and 10 and how.