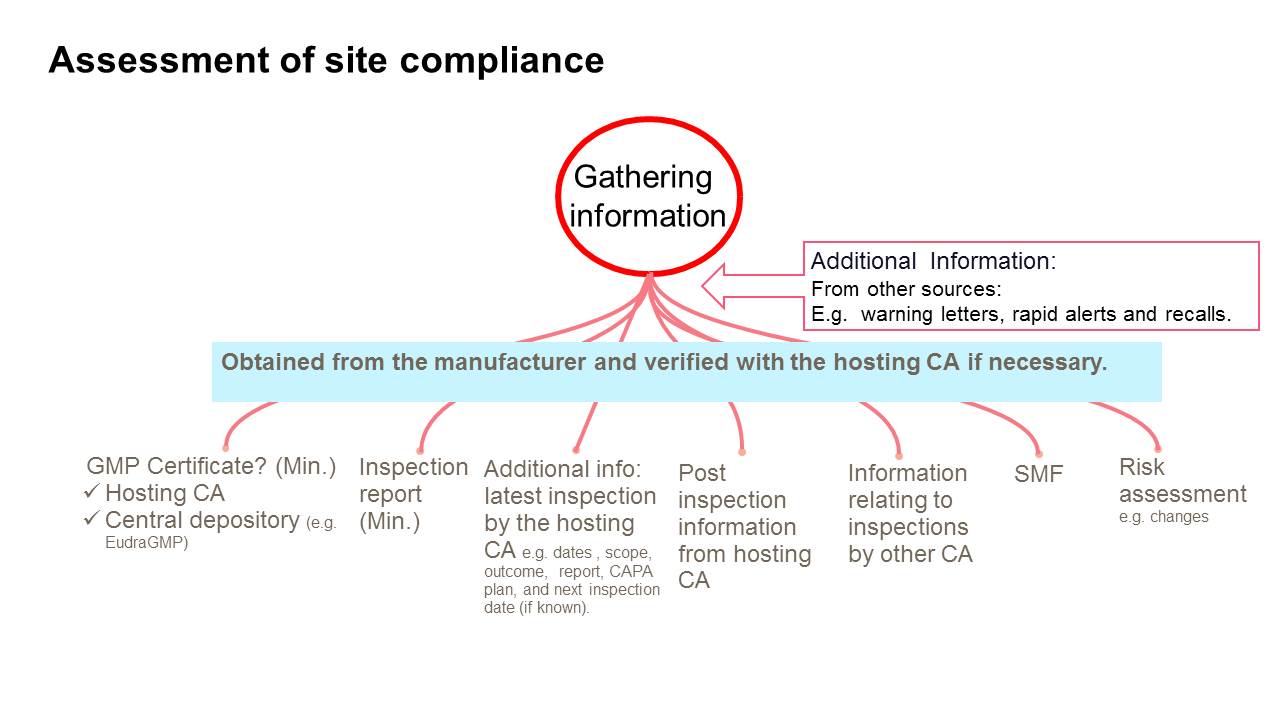
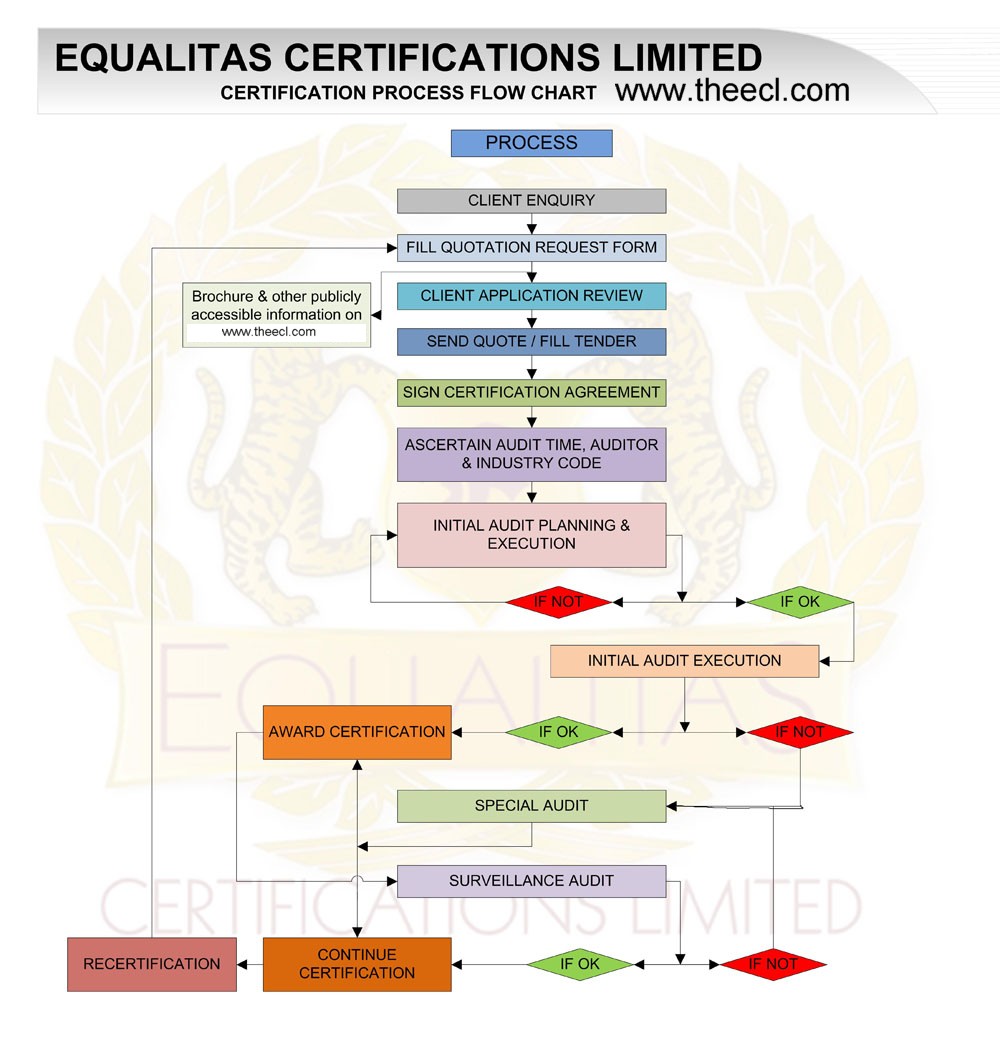
Gmp Certification Process

Good manufacturing practices gmp are the part of quality assurance that ensures that drugs are consistently produced and controlled in such a way to meet the quality standards appropriate to their intended use as required by the marketing authorizationpart of the health products and food branch inspectorate inspectorate program is to conduct inspections of establishments that are.
Gmp certification process. To achieve initial registration facilities are audited to nsfansi 173 section 8 which was developed in accordance with the fda regulation 21 cfr 111 on dietary supplement good manufacturing practices gmps. Good manufacturing practice gmp resources good manufacturing practice gmp is a system for ensuring that products are consistently produced and controlled according to quality standards. Cgmps provide for systems that assure proper design monitoring and control of manufacturing processes and. Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug administration under the authority of the federal food drug and cosmetic act.
Assurance of the safety and quality of food is an important consideration for consumers today. It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product. Pharmaceutical gmp professional certification cpgp process. Thinking that the procedure for a gmp certification is similar to that of an iso certification is a common failure ie.
Gmp certification and gmp certificate in europe an overview again and again we receive questions about gmp certification and gmp certificates. Nsf gmp registration is awarded to facilities that are in compliance with the standard nsfansi 173 section 8. Fda ensures the quality of drug products by carefully monitoring drug manufacturers compliance with its current good manufacturing practice cgmp regulations. Cgmp refers to the current good manufacturing practice regulations enforced by the fda.
Learn prepare apply certify recertify the certified pharmaceutical gmp professional understands the good manufacturing practices gmp as regulated and guided by national and international agencies for the pharmaceutical industry.