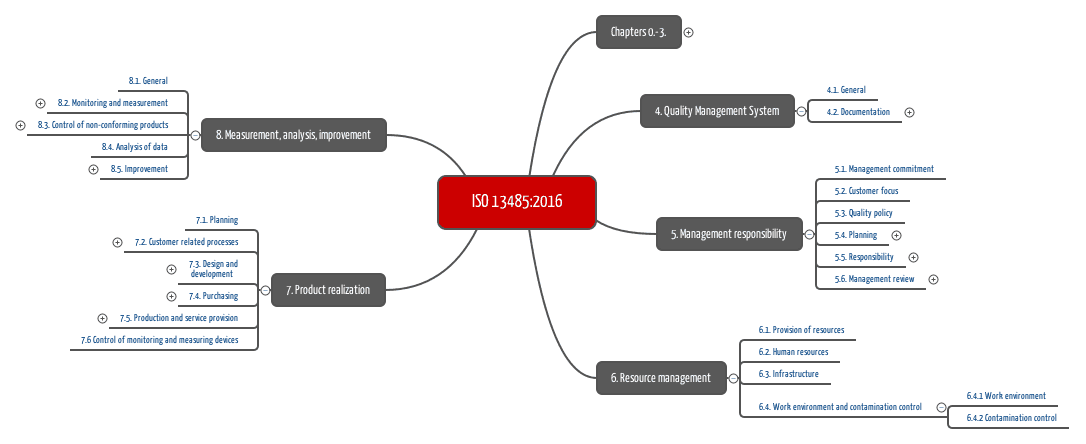
Iso 13485 Procedures Template

Iso 134852003 vs 2016 conversion tool.
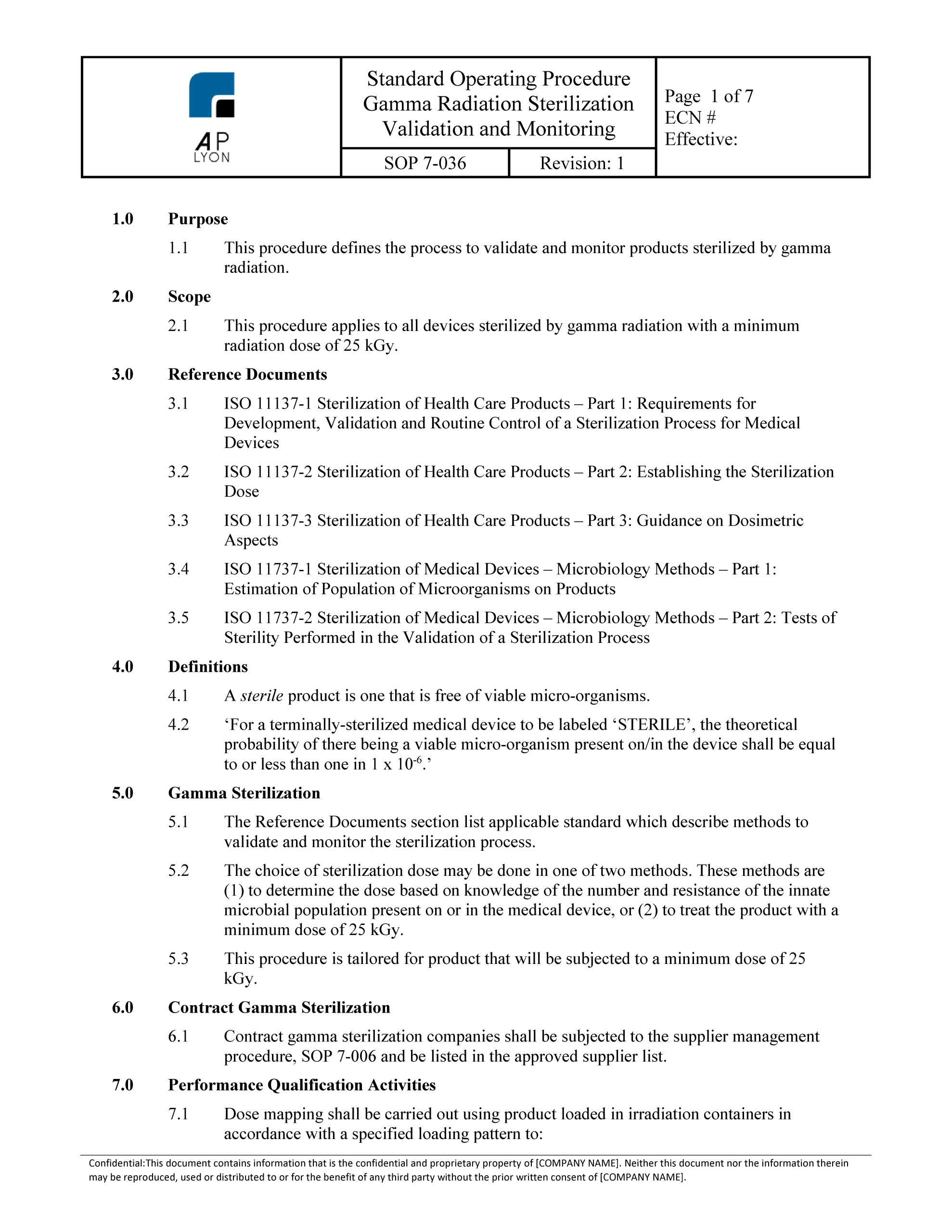
Iso 13485 procedures template. Just select the number of your current clause below and you will nd out which clause in iso 134852016 corresponds with it and what kind of changes do you need to perform in your quality management system for design and manufacture of medical devices to. Iso 134852016 mandatory procedures. This free tool will help you to convert iso 134852003 clauses to the new iso 134852016 clauses. The purpose of this procedure is to define the design control process used by the organization during the design and development of its products.
Quality management system templates covering both the iso 90012015 annex sl 10 section format and iso 134852016 8 section format in one combined annex sl manual. The template documentation covers both iso 134852003 and fda qsr 21 cfr part 820 requirements under one quality system and is thus ideally suited for companies that must comply with both the us fda and international regulations. Procedure for design and development. Iso 13485 document template.
Iso 13485 procedures with our free template version 2016 published by monir el azzouzi on july 6 2018 july 6 2018 have you ever been in this situation where you have to create the iso 13485 procedures or sops standard operating procedures and dont know where to start. Product information quotations and orders. Pack of iso 13485 templates includes quality management system templates for developing policies standard operating procedures sops and work instructions for the following areas of your business. If you plan to reconfigure your existing quality manual completely by yourself you can use either of the upgrade instructions to create everything on your own.














.jpg)