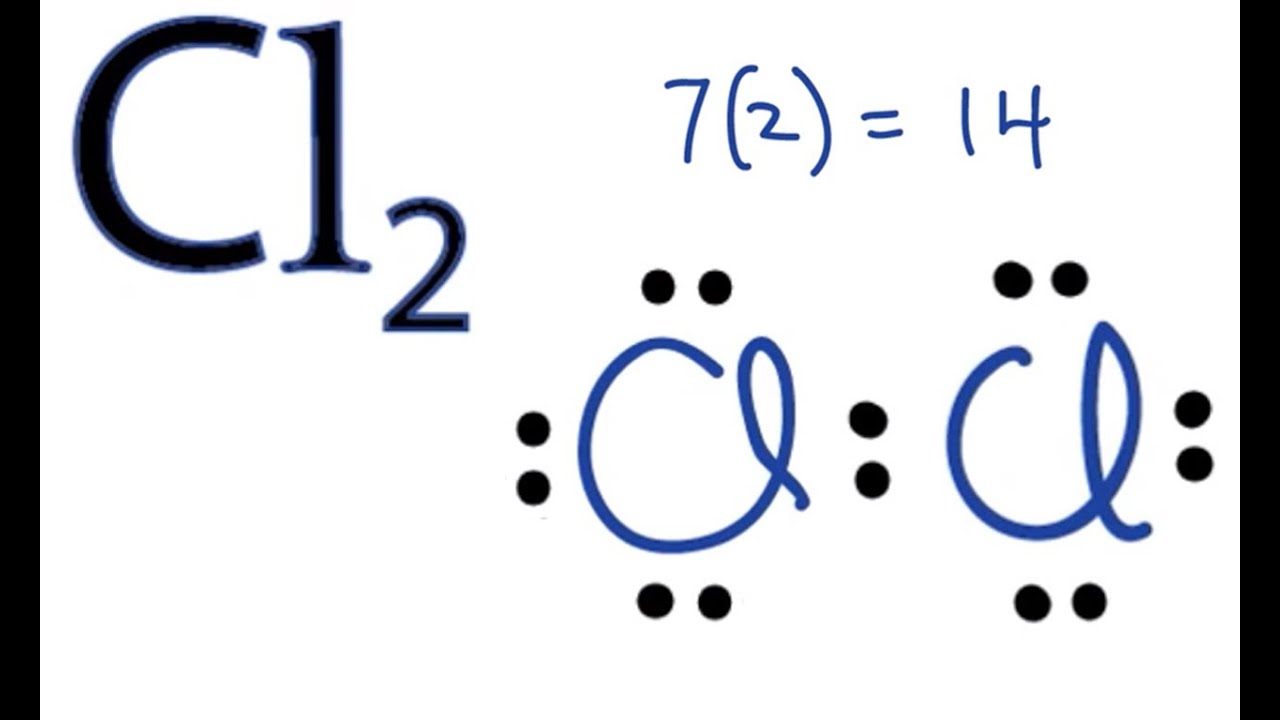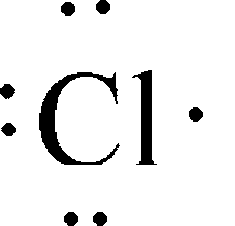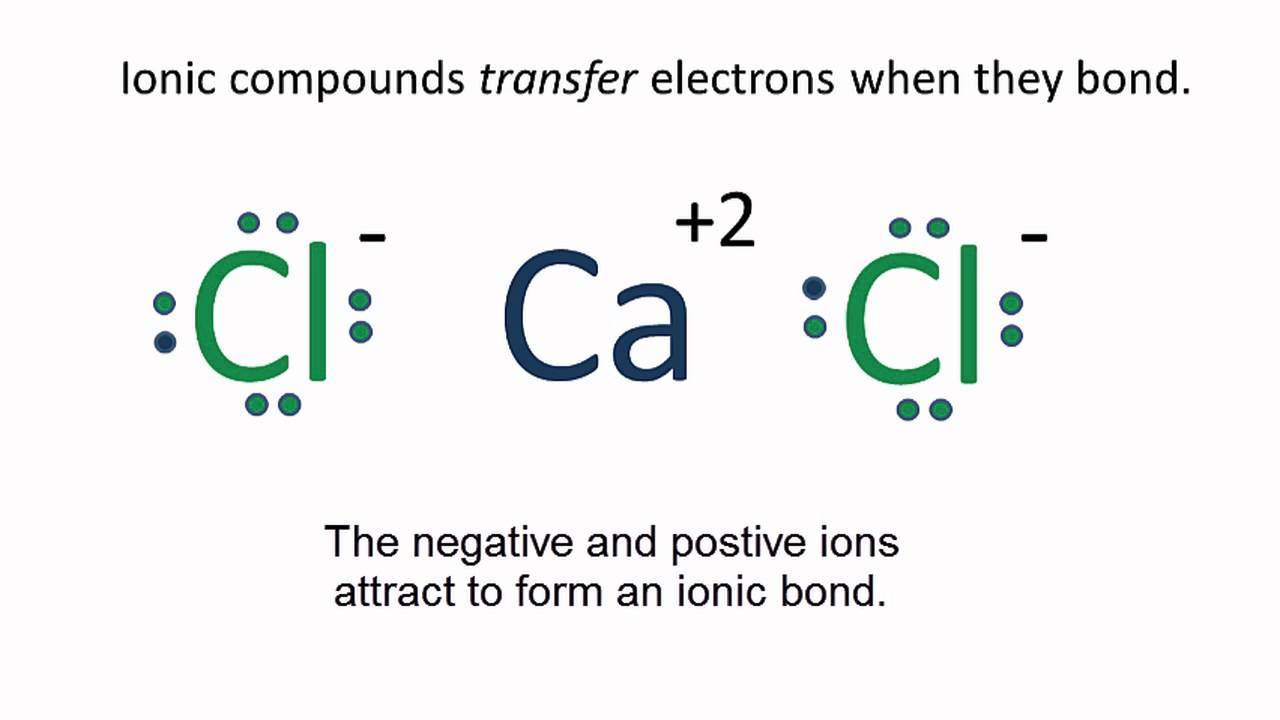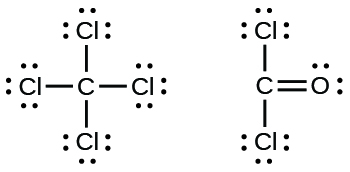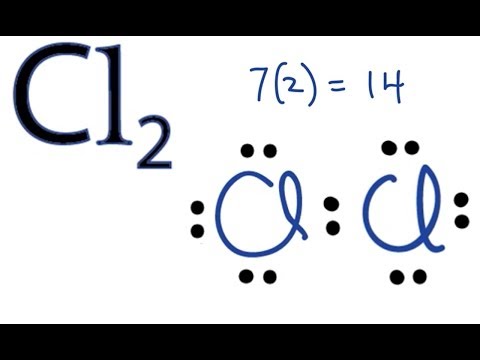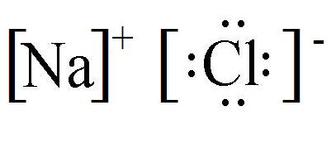Lewis Dot Diagram Chlorine Atom

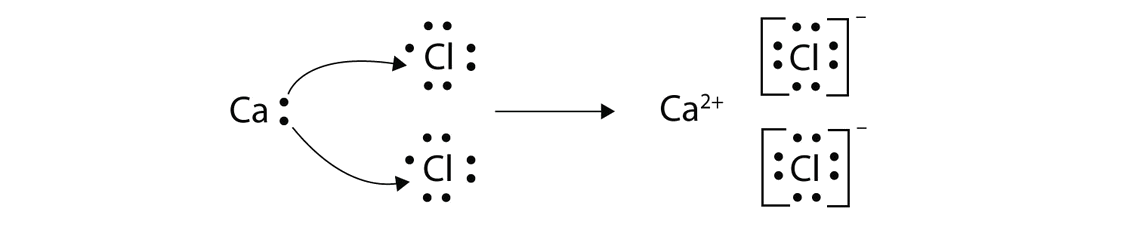
Because the chloride ion cl has an extra electron the negative sign denotes an extra electron we need to add that to the 7 valence electrons for the neutral cl atom.
Lewis dot diagram chlorine atom. Lewis structure electron dot diagram for hydrogen fluoride or the 2 electrons making up the bonding pair of electrons between the hydrogen atom and the fluorine atom which may or may not be circled are referred to as a covalent bond or a single covalent bond. The symbol for chlorine is cl. The lewis dot structure for chlorine will have a pair of electrons on each of three sides and just one electron on the fourth side. Each dot represents a valence electron each line represents a bonded pair of electrons and each cl represents a chlorine atom.
Chlorine like all halogens has seven dots in its lewis dot diagram. These are electrons in the outermost shell. For electron dot diagrams this symbol represents the nucleus and all of the electrons of the atom except the outermost electrons. Chlorine atoms have 7 valence electrons.
Lewis structures also known as lewis dot diagrams lewis dot formulas lewis dot structures electron dot structures or lewis electron dot structures leds are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. In an electron dot diagram this symbol represents the nucleus and the ten electrons in the first two energy levels. In the lewis structure of. After that i draw the lewis dot structure for chlorine cl.
A lewis electron dot diagram or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Chlorine is in group 17 sometimes called group vii or 7a. The number of dots equals the number of valence electrons in the atom.