Lithium Bohr Model Diagram

A bohr diagram is a simplified visual representation of an atom that was developed by danish physicist niels bohr in 1913.
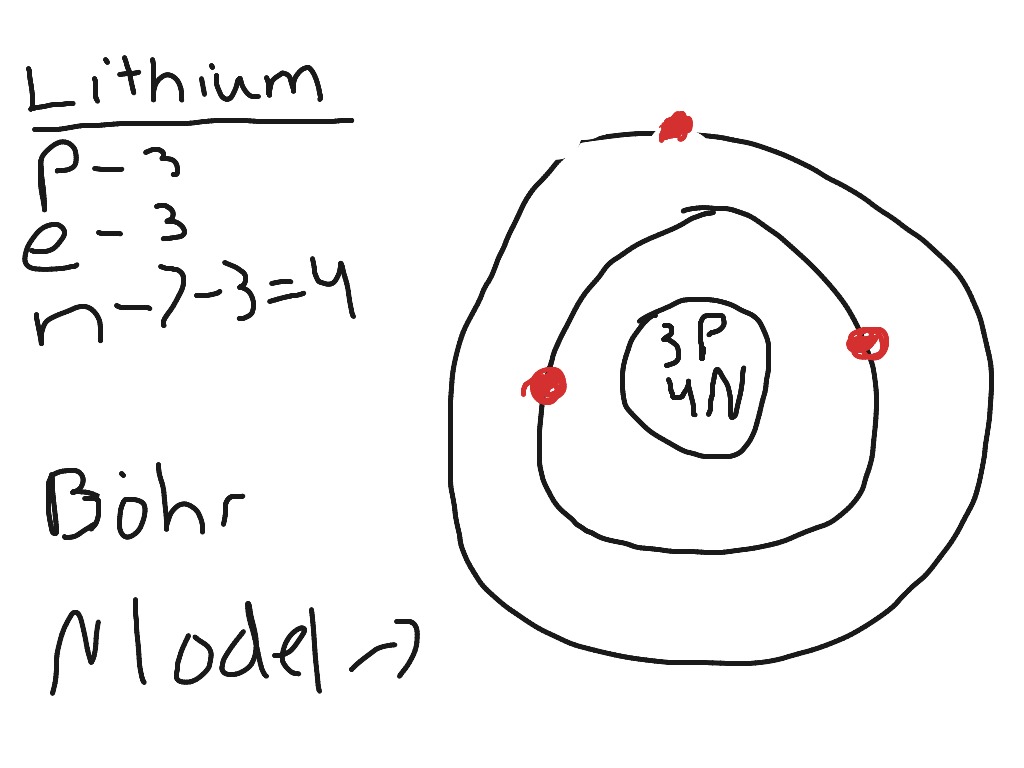
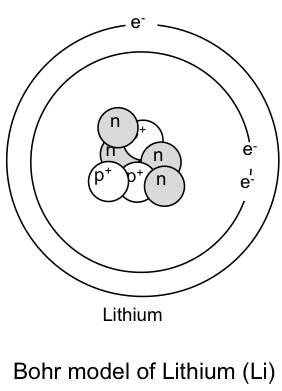
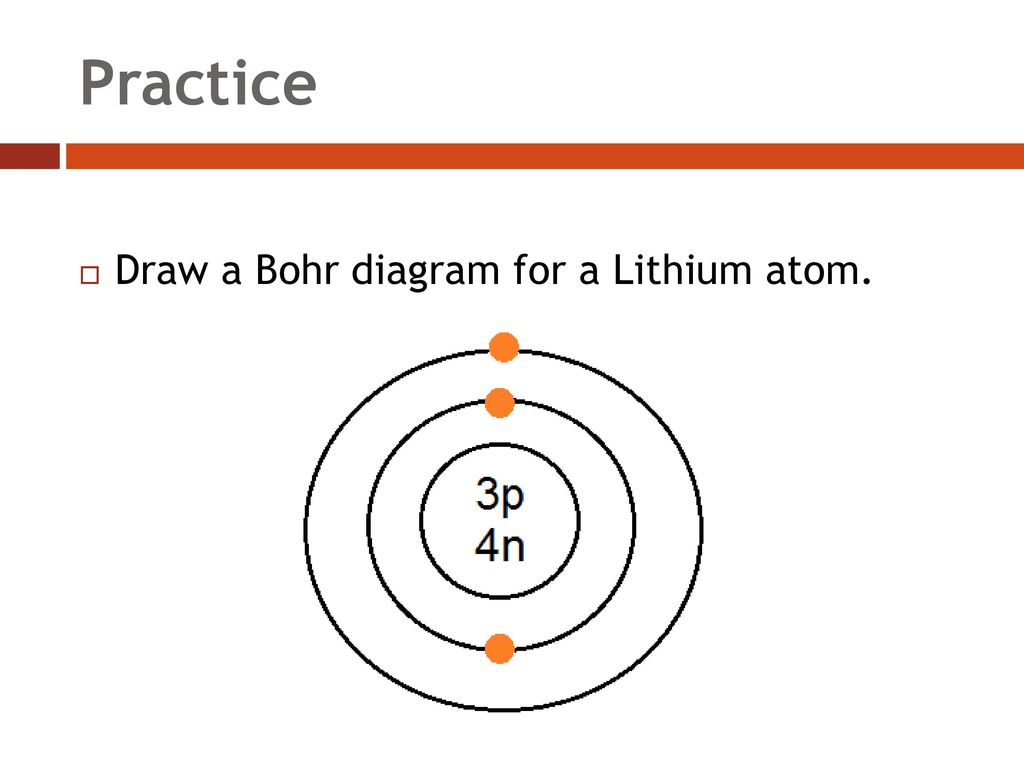
Lithium bohr model diagram. Bohr diagrams are used to introduce. Li in the centre then draw two circles around it then put two electrons on the inner circle and then 1 electron on the outside. What is the bohr model diagram for lithium. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.
Our new bohr model has suceeded in calculating the helium ionization energy more correctly than the quantum mechanical variational methods as shown in the top page. Unsubscribe from asal dariush. In the bohr model electrons are pictured as traveling in circles at different shells depending on which element you have. In the bohr model electrons have specific energy and can occupy specific shells.
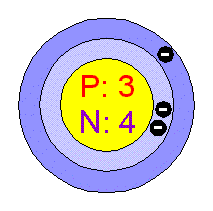
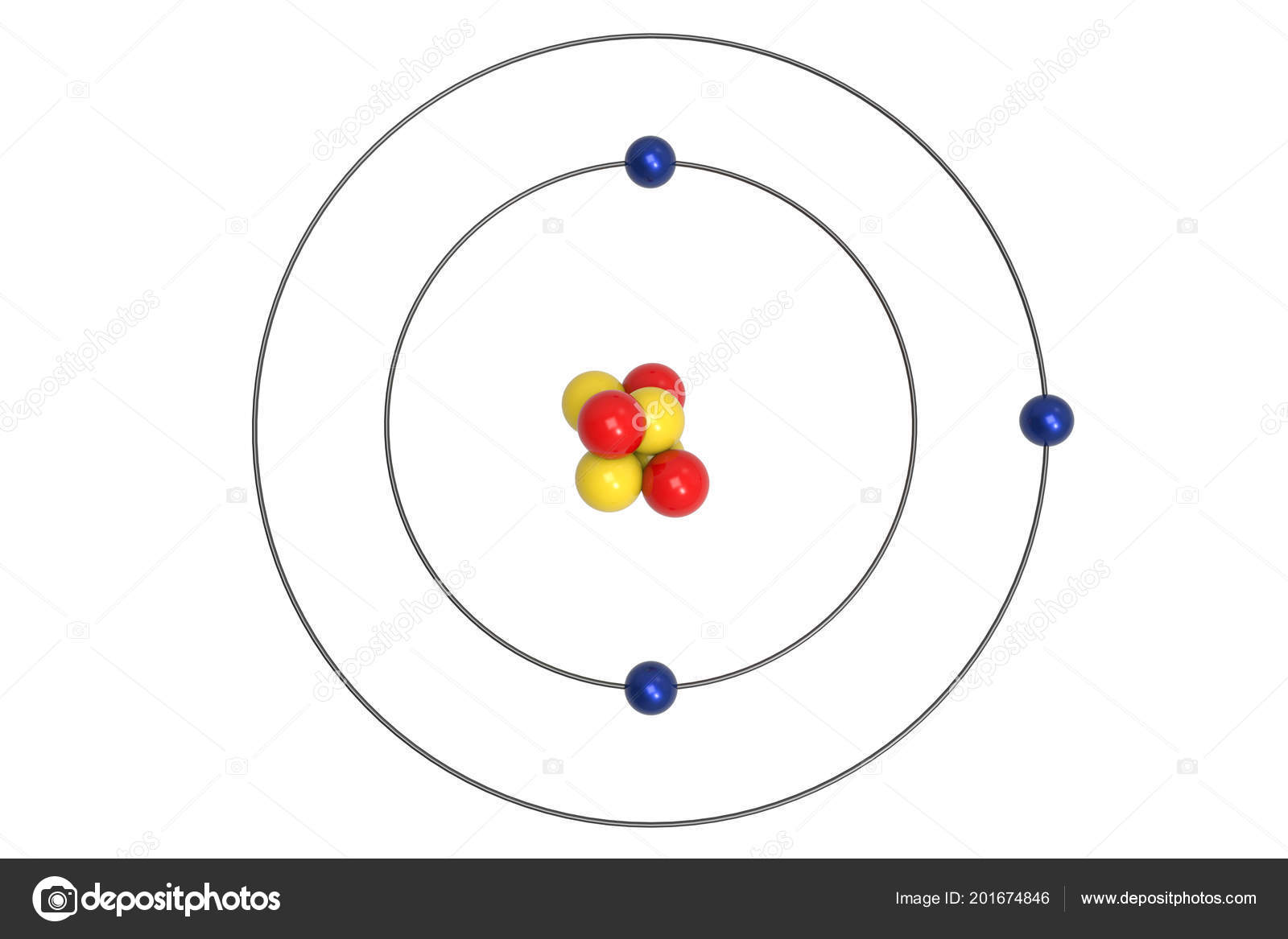
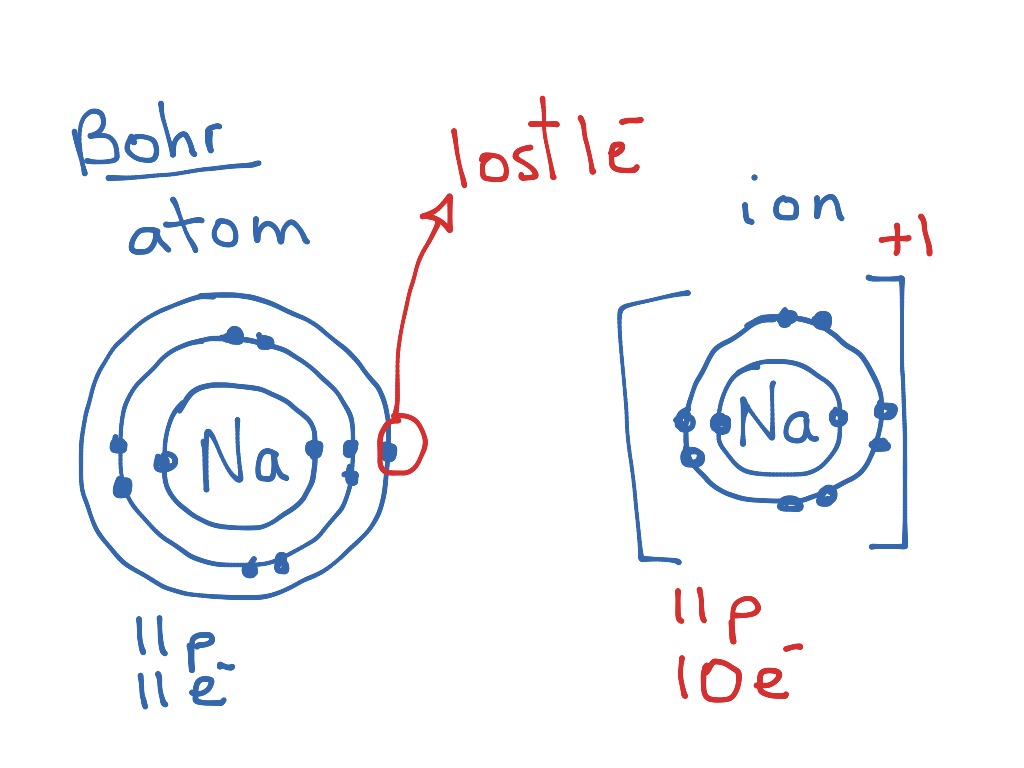
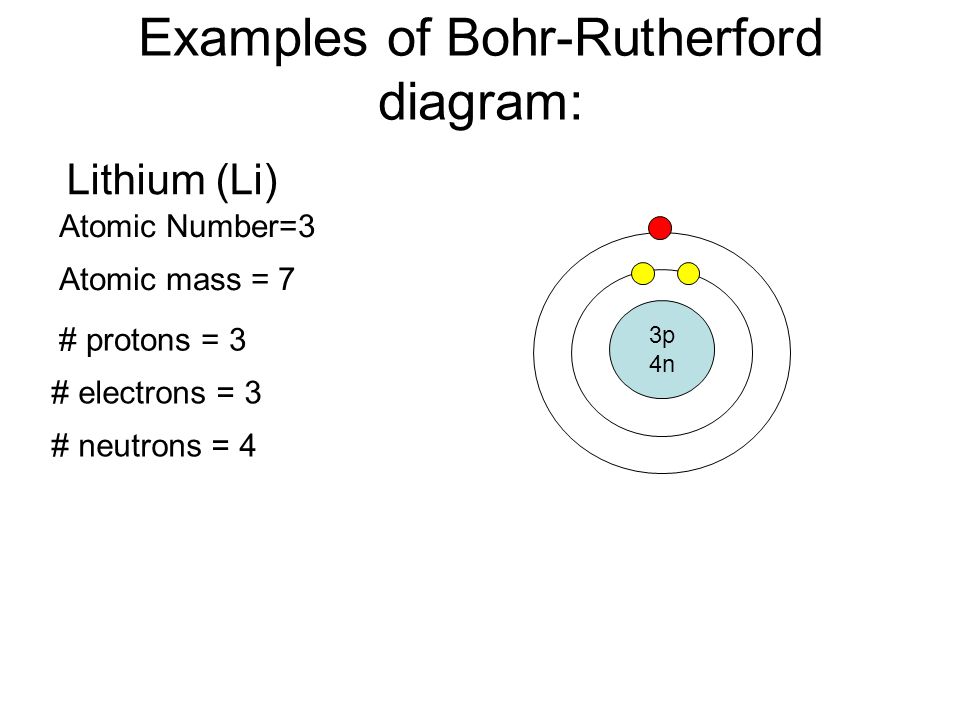
The bohr model of lithium contains a nucleus and two shells the first with two electrons and the second with one electron. Lithium belongs to the alkali metal group of chemical elements and has the atomic number 3. The bohr model of the atom was a planetary model. Bohr diagrams bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.
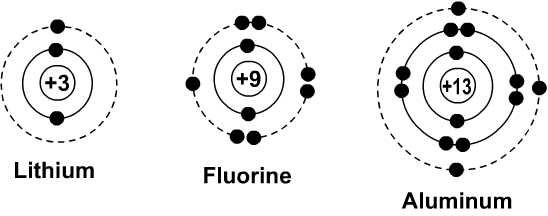
Figure pageindex2 contrast the bohr diagrams for lithium fluorine and aluminum atoms. Because of electron affinity the two electrons in the first shell are relatively close together. Bohr model of lithium asal dariush. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.
The following diagrams are for magnesium and lithium. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. How to draw bohr rutherford diagrams duration. Therefore the transition from one shell to an other is well defined.
This not only involves one electron systems such as the hydrogen atom singly ionized helium and doubly ionized lithium but it includes positronium and rydberg states of any atom where one electron is far away from everything else. Bohr rutherford diagram for lithium. In the bohr model electrons are pictured as traveling in circles at different shells depending on which element you have.