Mdr Technical Documentation Template
The technical documentation is going to be needed at some point for all devices.
Mdr technical documentation template. It was just good practice. Harmonized standards common specifications articles 8 and 9 mdr see annex ix x and xi of mdr for details. The new mdr dec. Assessment of technical documentation.
Declaration of conformity template mdr ivdr. Thank you for your question about the technical documentation. Technical documentation annex ii mdr 3. The new medical device regulation mdr has arrived.
Technical documentation for class iii and class iib implant products must be available in the english language. The primary language for all audit related documents is english. The regulations contain enhanced requirements for technical documentation often referred to as the technical file for each medical device or family and requires that the information be. 2379 page 1 of 4 the following structure is based on regulation eu 2017745 mdr but is also suitable for technical documentation according to di.
For products that still are under mdd because they have a certificate the doc can be changed when the requirements to mdr or ivdr. As soon as you update your technical documentation to make it compliant to the mdr or ivdr you should issue a new doc with the appropriate statement. This eu mdr technical documentation template will provide you all the necessary information that you need to gather. Annex ii and annex iii specify only that the technical documentation shall include in particular the elements listed in this annex.
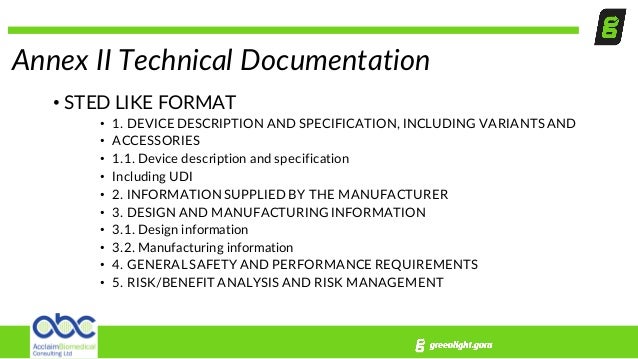
This technical documentation template conforms to eu mdr 2017745 annex ii and annex iii requirements. Mdr documentation submissions revision 1 september 2018 page 1 of 29 mdr documentation submissions. Best practices guidelines. First you need to know that the eu mdr 2017745 is providing a clear view of what should contain a technical file when the mdd 9342ec was not so structured.
Annexes ii and iii do not specify the structure or format of the technical documentation as is the case for example with the medicines ectd. Technical documentation requirements under mdr including requirements for your legacy files. Fortunately imrdf or ghtf created a template called sted summary technical documentation medical device to help organize all the information but this was not mandatory per legislation.



















