Medical Device Test Method Validation Template
What are medical device companies requesting these days when it comes to validation and verification.
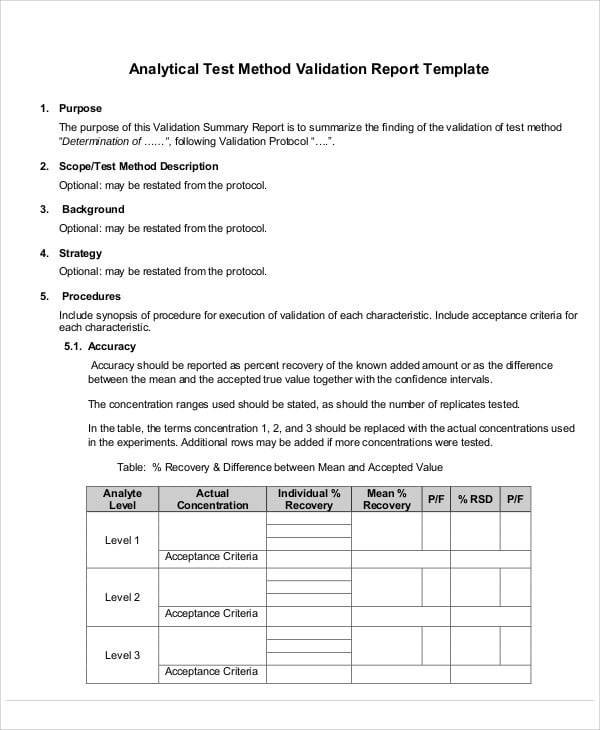
Medical device test method validation template. Demonstrating suitability must include attempting to quantify or classify the variances that. However regulatory expectation was evidenced by warning letters dating back to at least 2005 indicating that method validation is an applicable medical device validation activity for both physical and chemical test method. Identify known good and known bad products for all identified visual failure modes prior to performing product release testing delivery of templates for tm and tmv protocols and reports for medical devices creation and. Introduction test method validation is an often confusing requirement for medical devices.
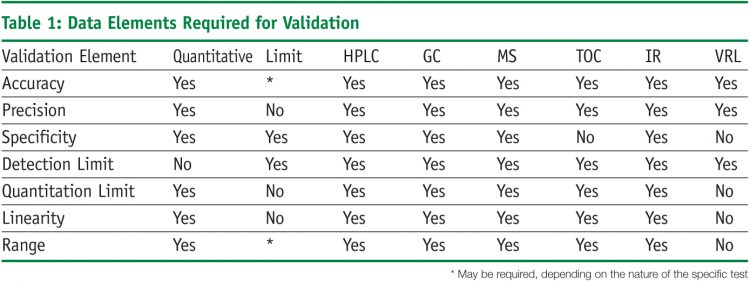
Measurement system analysis and test method validation. A fundamental issue is the role reversal between the test method and the product or process it is designed to detect. This is especially true in the medical device market. Medical devices where the resulting process output cannot be or is not verified by subsequent monitoring or measurement.
For example while a defect free process is desirable a test method must be reliable both in detecting defects and in not rejecting acceptable samples. In some instances validating methods is a regulatory requirement and an important element of quality control. Medical device testing guide. A critical difference in the previous installment of the blog we discussed our industries need to validate test methods in order to demonstrate that our measurement methods are capable of identifying variances inherent to production methods.
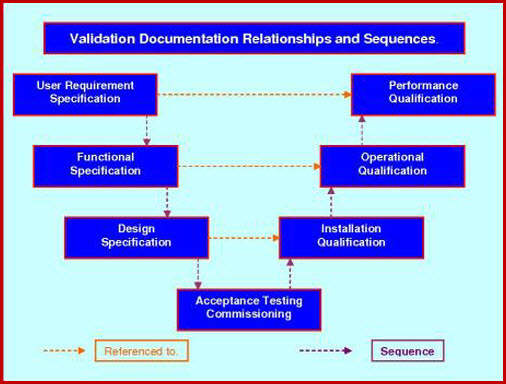
Template for process validation protocol contents point no. Although some traditional methods have been applied to chemical microbial and laboratory acceptance testing methods they are generally less utilized in the medical device industry. Section title pages no. More sophisticated testing and documentation tools for validation and verification will continue to evolve as medical devices become more complex.
Improved vv methods automated testing tools and documentation tools will undoubtedly. The medical device industry has long understood th method validation for medical devices regulatory guidance ivt method validation. For test method validation has been applied to chemical and to microbial acceptance test method. Test method validation case study.
Test method validation is an often confusing requirement for medical devices. Any time a new method is developed and validation methods are used in different test facilities methods should be validated.