Clinical Trial Protocol Template Ich

The address of the monitoring group i s listed below.
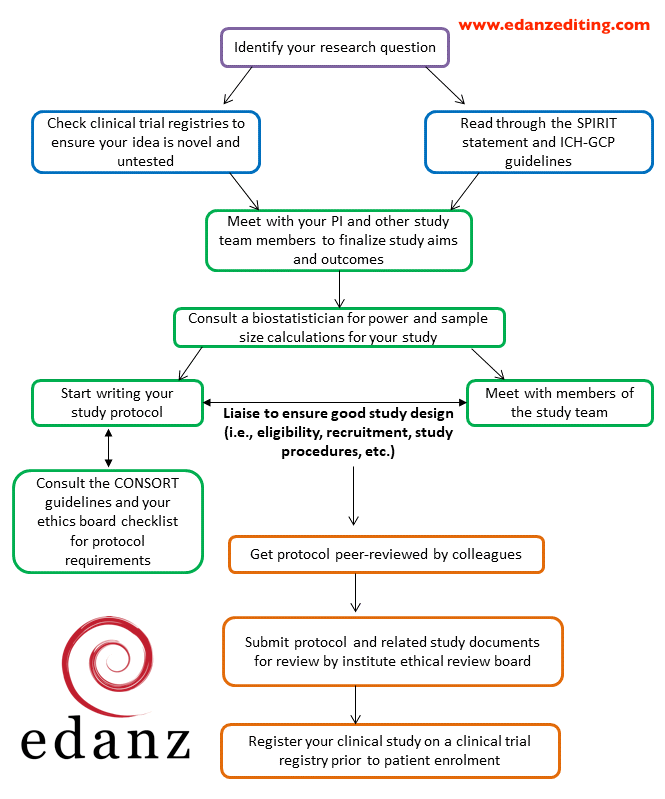
Clinical trial protocol template ich. However others may also find this template beneficial for other clinical trials not named here. The use of a standard protocol format this sample template was developed to assist protocol teams and principal investigators who are developing niaid daids supported andor sponsored clinical trial protocols. This template is provided to aid the investigator in writing a comprehensive clinical trial protocol that meets the standard outlined in the international conference on harmonisation ich guidance for industry e6 good clinical practice. The page is under construction.
The first type of trials are phase 2 and 3 clinical trial protocols that require a food and drug administration fda investigational new drug ind or investigational device exemption ide application. Using protocol templates you can start thinking through what you need to meet compliance. Every clinical investigation begins with the development of a clinical protocol. The contents of a trial protocol should generally include the following topics.
The protocol is a document that describes how a clinical trial will be conducted the objectives design methodology statistical considerations and organization of a clinical trial and ensures the safety of the trial subjects and integrity of the data collected. However site specific information may be provided on separate protocol pages or addressed in a separate agreement and. Suggested templates for phase 1 and 2 clinical trials. This study will be conducted in accordance with good clinical practice gcp requirements described in the current revision of international conference on harmonisation of technical requirements of pharmaceuticals for human use ich guidelines and all applicable regulations including current united states code of federal regulations cfr.
This protocol template aims to facilitate the development of two types of clinical trials involving human participants. Indide protocol word. This template provides a general format applicable to all clinical trials that are evaluating study productsinterventions. Generic protocol documents and instructions for ctep studies instructions for submitting protocol documents to ctep pdf step by step guide for submitting esubmission ready documents to ctep pdf generic protocol template ms word updated september 17 2019.
Its background rationale objectives design methodology statistical analysis plan and organizationwith the protocol you can make sure you protect the participants and collect the data.