Dietary Supplement Certifications

Ul offers a complete portfolio of services for the dietary supplement industry including quality assurance solutions regulatory compliance testing and global market access programs to meet consumer and regulatory demands for high quality dietary supplements.
Dietary supplement certifications. Some of our students finish in six months and those with work commitments may take a year or longer. Bscg offers a complete suite of certification testing and gmp compliance services to the dietary supplement and natural product industries. Dietary supplement compliance program certification let regulatory compliance be your unique advantage with dses regulatory and quality compliance program. You can study for as many hours per week as your work and social life allows.
Now was one of the first companies to be certified by underwriters laboratories ul for its dietary supplement manufacturing process following inspection of its good. Choose type of certification. The purpose of the ul national brand certification program nbcp is to assess the extent to which an organization conforms to the applicable regulations andor standards regarding the products being manufactured. The gold standard in dietary supplement certification.
Searching for nsf certified dietary supplements is quick and easy. Fda regulates dietary supplements under a different set of regulations than those covering conventional foods and drug products. Enter at least three letters of product model or product id. Fda regulates both finished dietary supplement products and dietary ingredients.
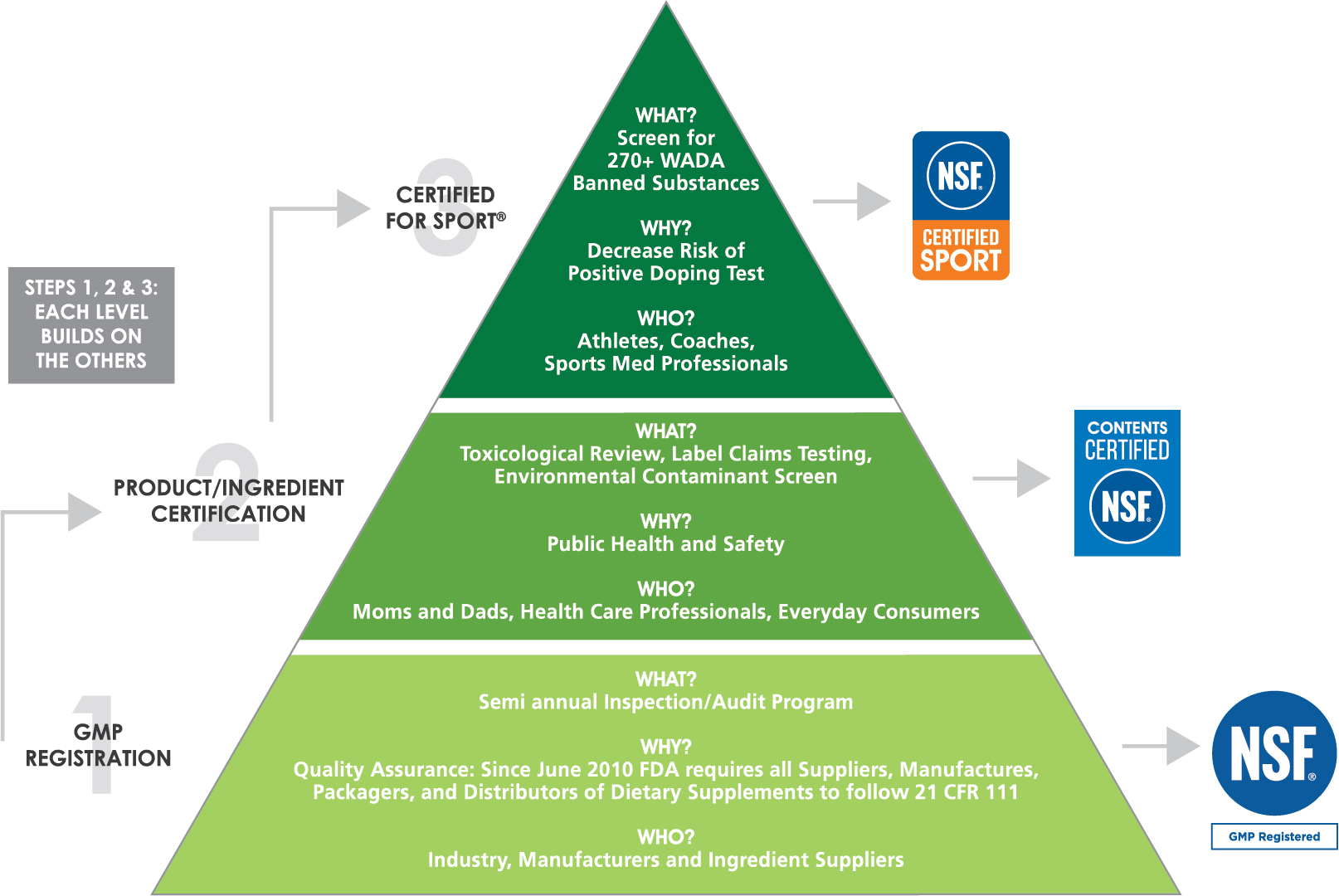
Dietary supplements the official american national standard for dietary supplement products. The guided learning hours to complete this training are 90 hours. Finally the highest level of independent third party certification requires testing on a lot by lot basis for more than 245 athletic banned substances. For more info visit nsf dietary supplements program.
Under the dietary supplement health and education act of 1994. If you have any problems please contact nsf. The certification process includes a toxicology label and formulation review to identify and quantify dietary ingredients declared on the product label a contaminant screen testing of declared supplement facts panel label claims lab testing to ensure the product does not include unsafe levels of contaminants and a good manufacturing. Dietary supplement testing and quality assurance.
Enter at least three letters of a manufacturers. Next supplement manufacturers can certify their products to nsfansi 173. Be prepared to weather the storms that your competitors will ultimately get caught in.