Iso 13485 Quality Manual Template
Use our free iso 13485 procedure template and the list of iso 134852016 mandatory procedures to build your medical device quality system and get certified.
Iso 13485 quality manual template. The medical device qms templates are used by our consultants in the field and are full of practical guidance and how to instructions. Kwikcert provides iso 13485 quality manual document template with live expert support. If you plan to reconfigure your existing quality manual completely by yourself you can use either of the upgrade instructions to create everything. We provide 100 success guarantee for iso 13485 certification.
This quality manual template is provided to you for free so dont hesitate to download it. This is the way i simplify it. But dont stick to the full content as everything is a fiction. Quality management system templates covering both the iso 90012015 annex sl 10 section format and iso 134852016 8 section format in one combined annex sl manual.
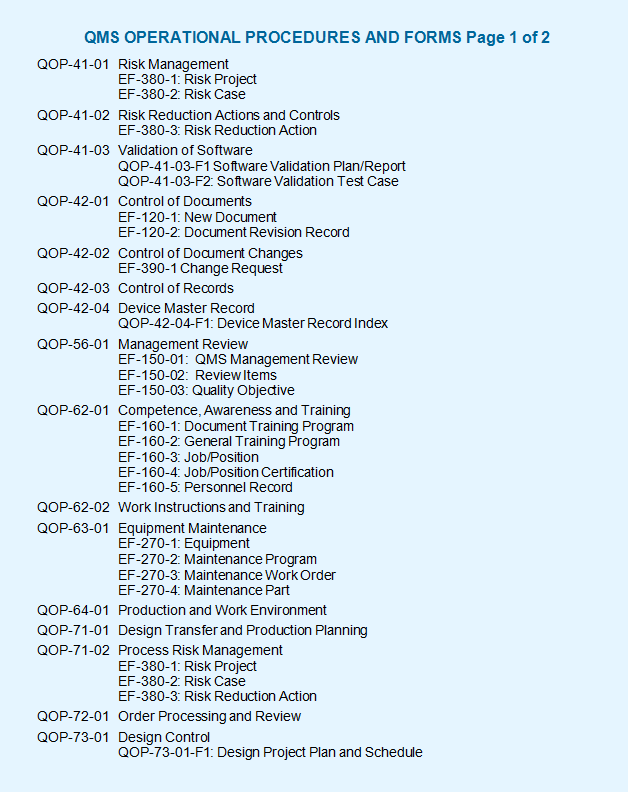
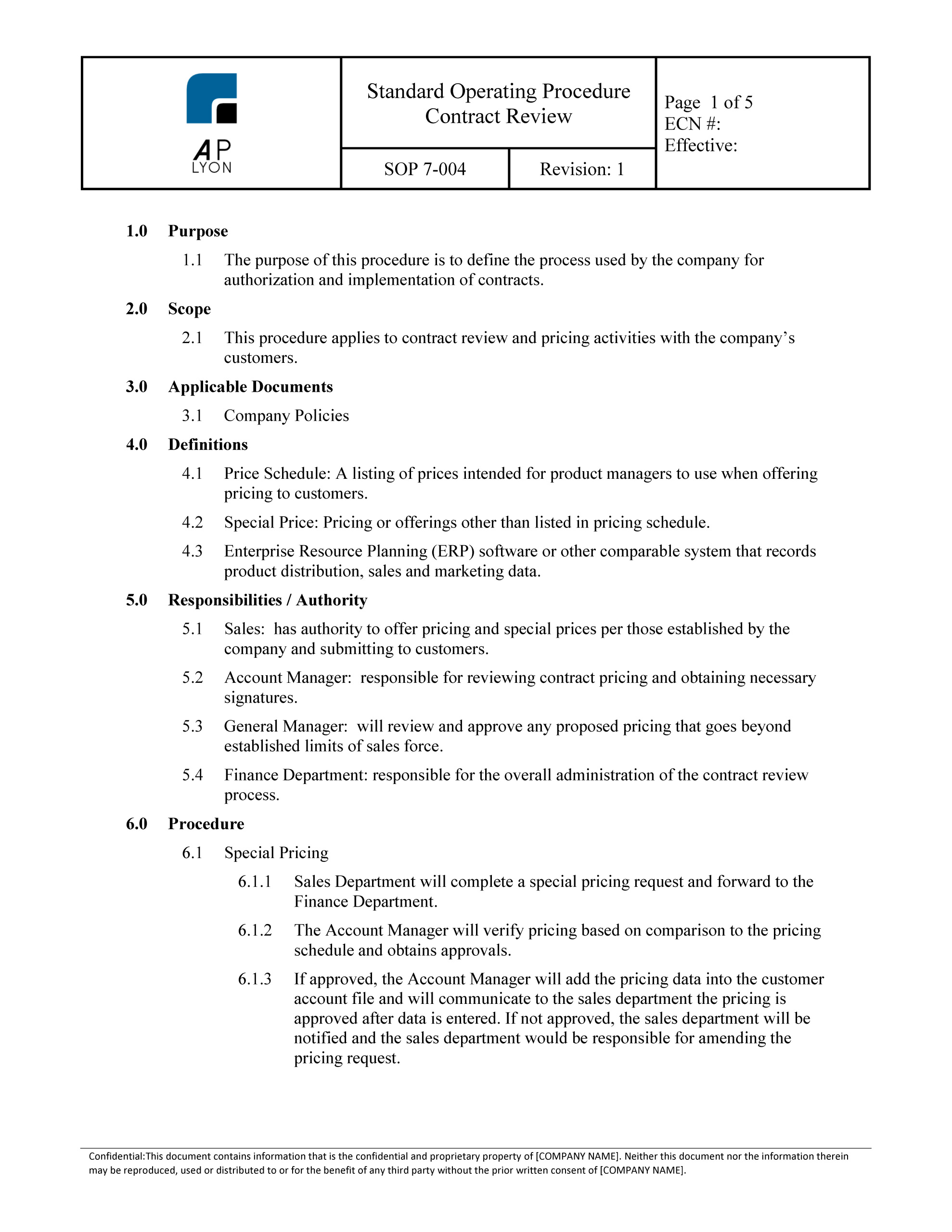
Built in microsoft word for easy editing these medical device qms templates are the quick and easy way to build a quality management system qms compliant with the iso 13485 standard or qsr 820 regulations. The iso 13485 documentation is a very easy process if it is developed with the basic knowledge of iso 13485 documents and medical devices in quality management system qms. The iso 13485 documentation kit include are iso 13485 quality manual procedures for quality management system exhibits and sops sample format and forms maintaining record process flow chart as well as iso 13485 audit. The quality manual demonstrates the capability of the organization to continuously provide products and services that address customer requirements.
I even created an iso 13485 quality manual example so you can see how short it can be. This is a description of what your company does eg distribution of medical devices software design for mri machines etc and the boundaries of your quality. Use of templates is a widely used approach to establishing and documenting quality management systems. 1 the qms scope.
To begin well look at the requirements of a quality manual and the thinking behind each requirement. By using this document you can implement iso 13485 yourself without any support. Do it yourself iso 13485 or qsr 820 compliance. The template documentation covers both iso 134852003 and fda qsr 21 cfr part 820 requirements under one quality system and is thus ideally suited for companies that must comply with both the us fda and international regulations.
Imsxpress iso 13485 template documentation is part of imsxpress iso 13485 software. I made it like if easy medical device is a company manufacturing medical device products. The document is optimized for small and medium sized organizations we believe that overly complex and lengthy documents are just overkill for you. What does iso 13485 require from the quality manual.