Medical Device Quality Management System Template

Medical device quality system templates.
Medical device quality management system template. All files are provided in an editable ms word or excel format. Complete medical device qms template package. Med dev qms provides iso 134852016 and fda qsr compliant quality system templates specifically developed for startup small medical devices firms. We all have a standard template for our quality system procedures.
Do it yourself iso 13485 or qsr 820 compliance. How to write a quality system plan template free download posted by rob packard on november 20 2015. Iso 13485 is the standard for medical device companies. This article explains how to write a quality system plan template to revise and update your quality system for compliance with iso 134852016 when it is released early next year.
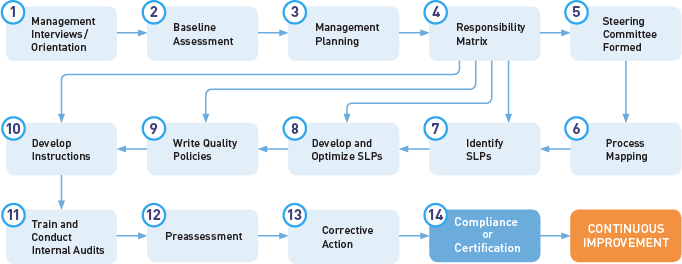
81500 quality manual procedures forms a quality manual 28 quality procedures 16 quality forms and 24 additional templates and logs to get your quality management system implemented quickly and effectively. If you have one to know it should be this one. The medical device qms templates are used by our consultants in the field and are full of practical guidance and how to instructions. A new 12 part procedure template for your medical device qms posted by brigid glass guest blogger on october 7 2013.
The name of this standard is. A quality system and here is why we need iso. Let us help you focus on getting products to market faster. Because of this a medical device quality system often gets pushed to the back burner in favor of activities that are perceived to add more value and meaning to the startup.
Typically we begin with purpose scope and definitions. Medical device startups have a pile of things to address yet often dont have the pile of money to take care of everything that is needed.