Fda Device Master Record Template
21 cfr 820181 device master record explains that each manufacturer shall maintain dmrs including preparation and approval per 21 cfr 82040.
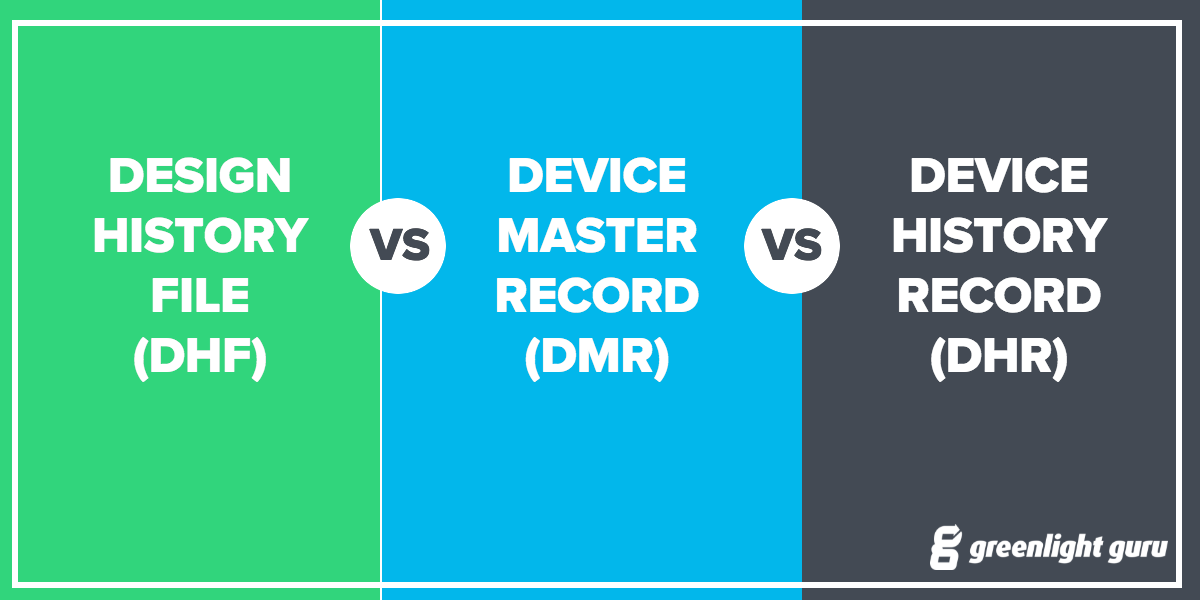
Fda device master record template. The total finished design output consists of the device its packaging labeling and the device master record. The food and drug administration fda requires manufacturers of medical devices to create and maintain a device master record dmr. Design history file dhf vs. Each manufacturer shall maintain device master records dmrs.
Consulting firm providing cost effective fda regulatory affairs services world wide. Section 8203j of the federal code defines device master record. The total finished design output consists of the device its packaging and labeling and the device master record. Understanding the differences and what documents to include by jesseca lyons july 25 2016 in fda regulations and product development and design controls.
Master files help preserve the trade secrets of the ancillary medical device industry and facilitate the sound scientific evaluation of medical devices. Record of critical operations in device history records by responsible individual. The finished design output is the basis for the device master record dmr. Dmr is a set of documents containing procedures and specifications for a finished medical device.
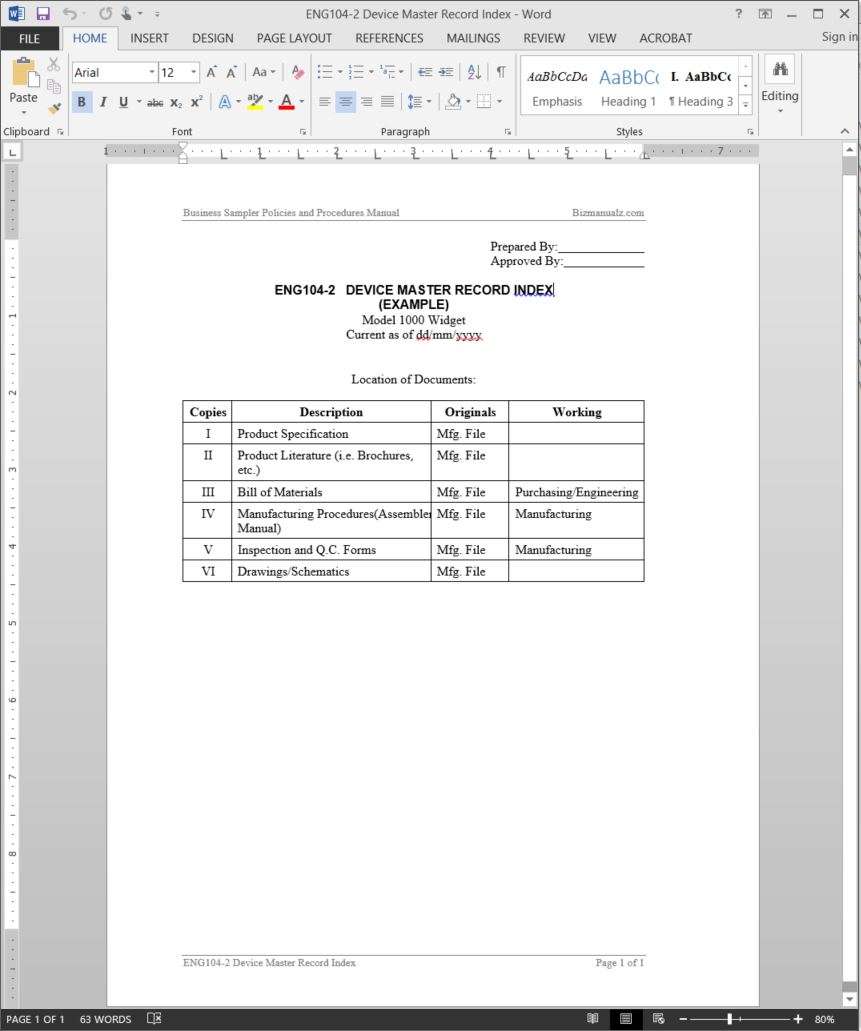
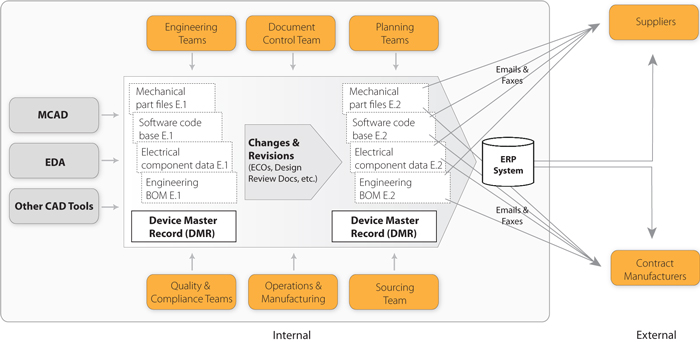
The dmr for each type of device shall include or refer to the location of the following information. Device master record dmr vs. Eng104 2 device master record index includes descriptions such as product specification and product literature. Device history record.
The dmr for each device type shall include or refer to the. Device history record dhr. A master file is a service for your customers but also helps you to maintain control on your companys proprietary information. Upon compilation of all documents to be included in the master record a device master record index template should be prepared to identify all items in the record and locations of these records.
Each manufacturer shall ensure that each dmr is prepared and approved in accordance with 82040.