Clinical Research Protocol Template

Clinical trials protocol templates.
Clinical research protocol template. Clinical study report template. Applicants conducting phase 2 or 3 clinical trials that require investigational new drug applications ind or investigational device exemption ide applications can use a nih fda template with instructional and sample text to help write protocols. Clinical data to date. Rationale for this study.
Dose rationale and riskbenefits. The research protocol is an essential part of a research project. It is a full description of the research study and will act as a manual for members of the research team to ensure everyone adheres to the methods outlined. A separate template is available for applicants conducting.
Investigator brochure or imp dossier development sop. Prior literature and studies. Risk assessment for trial sop. Purpose of the study protocol.
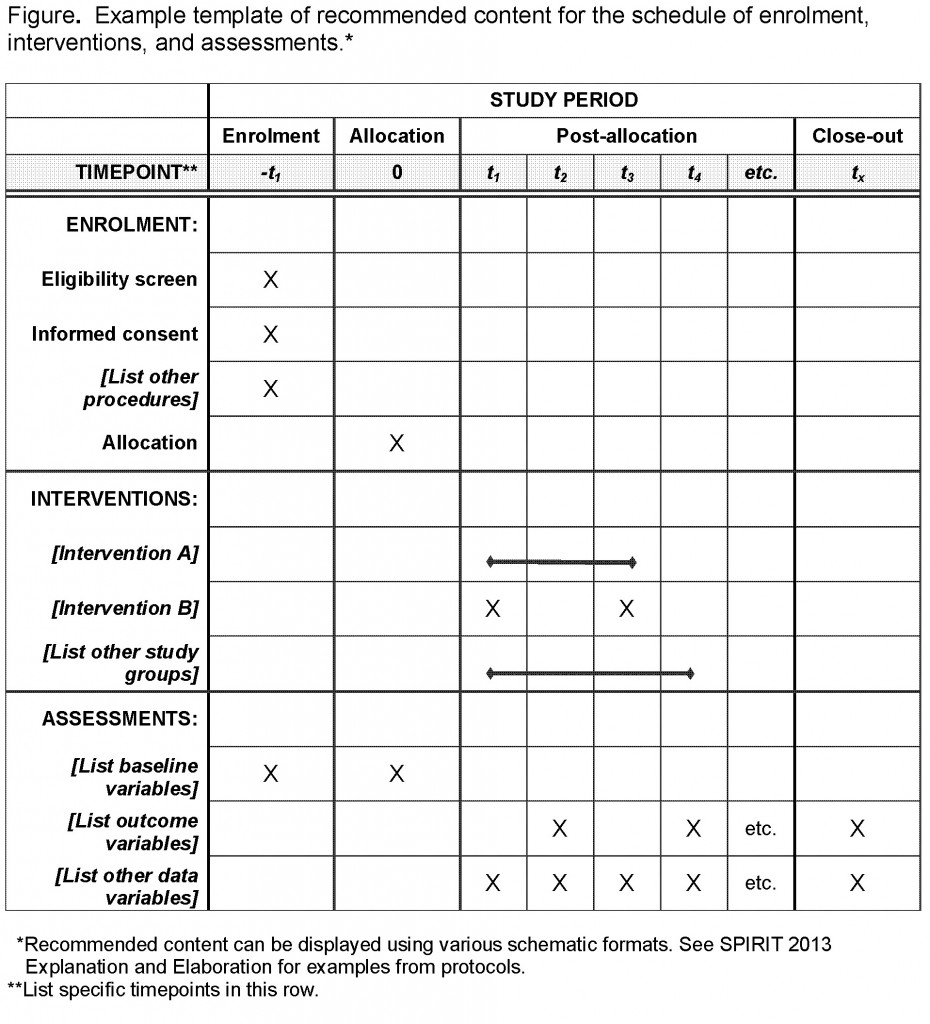
Templates for developing a manual of operating procedures moop guidelines and templates for developing a clinical study moop to facilitate consistency in protocol implementation and data collection across staff patients and clinical sites. The protocol is a document that describes how a clinical trial will be conducted the objectives design methodology statistical considerations and organization of a clinical trial and ensures the safety of the trial subjects and integrity of the data collected. Protocol feasibility assessment sop. Nhlbi sample protocol template september 2006.
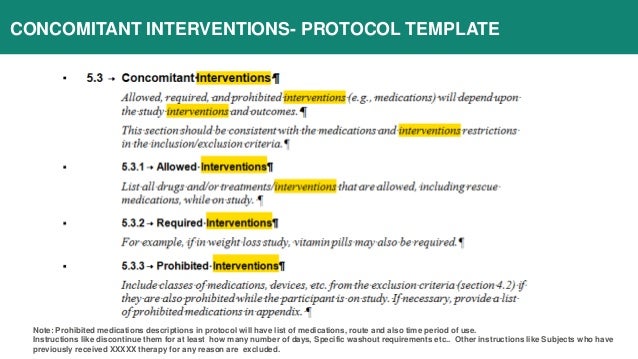
Download the template s of your choice. The intervention template is ich gcp compliant. Protocol templates and guidelines suggested templates for phase 1 and 2 clinical trials. Protocol amendments assessment sop.
None of the templates is likely to be perfect for a given study without some modification. They follow the format of typical nih and industry multicenter protocols. Prmc tissue research submission template. As the study gets underway it can then be used to monitor the studys progress and evaluate its outcomes.
The following templates are primarily designed for studies focused on. Every clinical investigation begins with the development of a clinical protocol. The following guidelines and templates ensure consistent operating procedures and oversight for clinical research studies. The irb provides several protocol templates on this page.
Concept protocol template. Investigators for such trials are encouraged to use this template when developing protocols for nih funded clinical trials. It is expected that the investigator will adapt the template to suit their needs.